86040
Acido sulfamico
analytical standard (for acidimetry), ACS reagent
Sinonimo/i:
Acido amidosolfonico
About This Item
Prodotti consigliati
Grado
ACS reagent
analytical standard (for acidimetry)
Livello qualitativo
agenzia
complies with DIN 19266
Saggio
99.3-100.3% (dried material)
Punto di fusione
215-225 °C (dec.) (lit.)
Anioni in tracce
chloride (Cl-): ≤10 mg/kg
sulfate (SO42-): ≤500 mg/kg
Cationi in tracce
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤10 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
applicazioni
environmental
food and beverages
general analytical
industrial qc
pharmaceutical
Formato
mixture
Stringa SMILE
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Sulfamic acid also finds its use as a primary standard in non-aqueous visual, conductometric, and potentiometric titrations.
Caratteristiche e vantaggi
- Available in a secure glass bottle to ensure its stability for the entire shelf life until opened.
- High-quality offering accurate titer determinations
- Accompanied by a detailed certificate of analysis (CoA)
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
8B - Non-combustible, corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.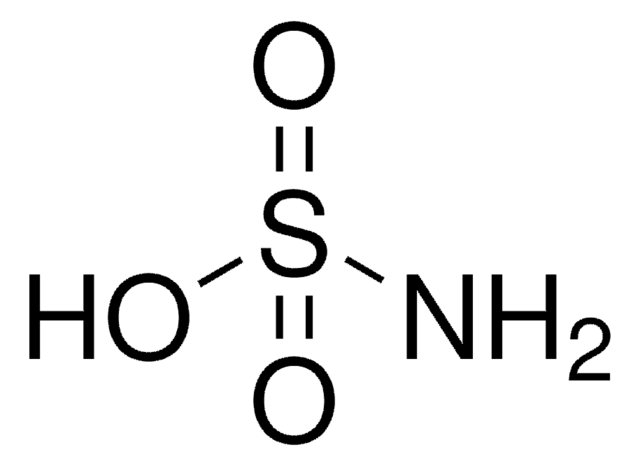


![[(3R)-3-Hydroxytetradecanoyl]-L-carnitine-(N-methyl-d3) analytical standard](/deepweb/assets/sigmaaldrich/product/structures/359/244/1ce23698-2525-447b-8fab-501b73a8c5da/640/1ce23698-2525-447b-8fab-501b73a8c5da.png)




