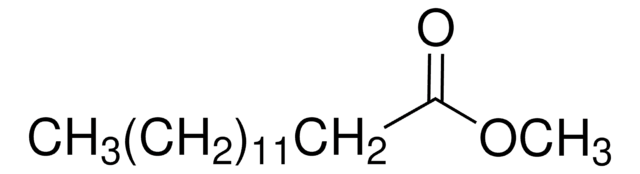70129
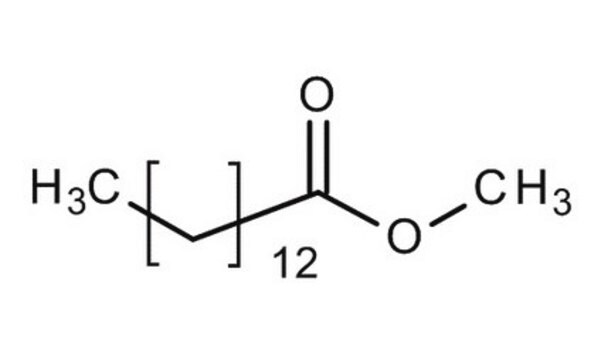
Methyl myristate
analytical standard
Sinonimo/i:
Methyl tetradecanoate, Myristic acid methyl ester
About This Item
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
Saggio
≥99.5% (GC)
Durata
limited shelf life, expiry date on the label
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.436 (lit.)
n20/D 1.438
P. ebollizione
323 °C (lit.)
Punto di fusione
18 °C (lit.)
Densità
0.855 g/mL at 25 °C (lit.)
Formato
neat
Gruppo funzionale
ester
Condizioni di spedizione
ambient
Temperatura di conservazione
room temp
Stringa SMILE
CCCCCCCCCCCCCC(=O)OC
InChI
1S/C15H30O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h3-14H2,1-2H3
ZAZKJZBWRNNLDS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Comparative analysis of gas chromatography-combustion-mass spectrometry and gas chromatography-flame ionization detector methods for the determination of fatty acid methyl esters (FAMEs) in biodiesel samples
- Gas chromatography-tandem differential mobility spectrometry (DMS) based separation and quantification of 16 methyl- and ethyl- fatty acid esters from biodiesel samples
- Analysis of coffee oil and residue obtained from roasted coffee beans to determine the composition of 11 fatty acids following their methyl esterification by gas chromatography coupled with a flame ionization detector (GC-FID)
- Simultaneous determination of fatty acids in bovine colostrum samples by GC-FID after their derivatization to ester forms using an acidic catalyst boron trifluoride
- Separation and quantification of eight fatty acids after their derivatization to methyl esters in the oil extracted from the leaves of Abutilon hirtum (Lam.) by GC-MS
Altre note
Prodotti consigliati
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
233.6 °F - closed cup
Punto d’infiammabilità (°C)
112.0 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
Separation of Methyl erucate; Methyl palmitate; Methyl stearate; Methyl linolenate; Methyl eicosenoate; Methyl behenate; Methyl myristate; Methyl oleate; Methyl arachidate
Protocol for GC Analysis of Bacterial Acid Methyl Esters (BAMEs) on Equity®-1
Separation of Methyl decanoate; Methyl dodecanoate; Methyl myristate; Methyl palmitate; Methyl caprylate; Methyl oleate; Methyl linoleate; Methyl linolenate; Methyl stearate
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.