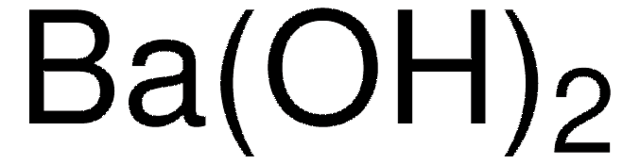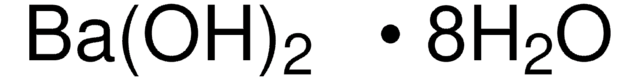1.01737
Barium hydroxide octahydrate
for analysis EMSURE® ACS,ISO,Reag. Ph Eur
Sinonimo/i:
Barium hydroxide octahydrate, Caustic baryta, Barium oxide hydrate octahydrate
About This Item
Prodotti consigliati
Grado
ACS reagent
Livello qualitativo
agenzia
reag. ISO
reag. Ph. Eur.
Nome Commerciale
EMSURE®
Saggio
≥98.0% (acidimetric)
Stato
solid
Potenza
550 mg/kg LD50, oral (Rat)
Impurezze
≤0.005% Substances insoluble in dilute hydrochloric acid
pH
14 (20 °C in H2O, saturated aqueous solution)
Punto di fusione
78 °C
Densità
2.18 g/cm3 at 20 °C
Densità bulk
900‑1100 kg/m3
Anioni in tracce
carbonate (as BaCO3): ≤2.0%
chloride (Cl-): ≤0.001%
sulfide (S2-): ≤0.0005%
Cationi in tracce
Ca: ≤0.002%
Fe: ≤0.0005%
K: ≤0.01%
Na: ≤0.01%
Pb: ≤0.001%
Sr: ≤0.5%
heavy metals (as Pb): ≤0.0005%
Temperatura di conservazione
2-30°C
Stringa SMILE
[Ba+2].[O-H].[O-H].O.O.O.O.O.O.O.O
InChI
1S/Ba.10H2O/h;10*1H2/q+2;;;;;;;;;;/p-2
ZUDYPQRUOYEARG-UHFFFAOYSA-L
Applicazioni
- Determination of phosphorylated and O-glycosylated sites by chemical targeting (CTID) at ambient temperature.: This study explores the use of chemical targeting to identify phosphorylated and O-glycosylated sites, essential for understanding protein modifications. Barium hydroxide is utilized for its effectiveness in ambient temperature conditions, contributing to more efficient analytical methods (Hathaway, 2007).
- Wet-chemical synthesis of crystalline BaTiO3 from stable chelated titanium complex: formation mechanism and dispersibility in organic solvents.: The research demonstrates the wet-chemical synthesis of BaTiO3, highlighting the formation mechanism and its dispersibility. Barium hydroxide plays a crucial role in stabilizing the chelated titanium complex, making it a valuable component in material synthesis and analytical chemistry (Pramanik et al., 2006).
- Synthesis and analgesic effect of normorphine-3- and -6-glucuronides.: This paper details the synthesis of normorphine glucuronides, where barium hydroxide is used in the synthetic process. The study contributes to the understanding of analgesic properties and the chemical synthesis of pharmacologically relevant compounds (Oguri et al., 1989).
Risultati analitici
Substances insoluble in dilute hydrochloric acid: ≤ 0.005 %
Carbonate (as BaCO₃): ≤ 2.0 %
Chloride (Cl): ≤ 0.001 %
Sulphide (S): ≤ 0.0005 %
Heavy metals (as Pb): ≤ 0.0005 %
Ca (Calcium): ≤ 0.002 %
Fe (Iron): ≤ 0.0005 %
K (Potassium): ≤ 0.01 %
Na (Sodium): ≤ 0.01 %
Pb (Lead): ≤ 0.001 %
Sr (Strontium): ≤ 0.5 %
Corresponds to ACS,ISO,Reag. Ph Eur
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.