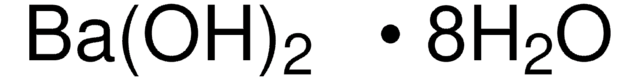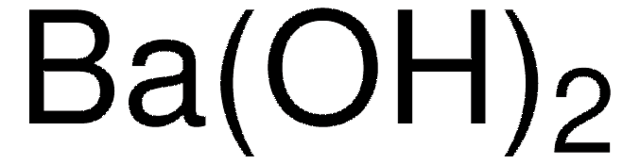1.01735
Barium hydroxide octahydrate
EMPLURA®
Sinonimo/i:
Barium hydroxide octahydrate, Caustic baryta, Barium oxide hydrate octahydrate
About This Item
Prodotti consigliati
Livello qualitativo
Nome Commerciale
EMPLURA®
Saggio
≥98.0% (acidimetric)
Stato
solid
Potenza
550 mg/kg LD50, oral (Rat)
Impurezze
≤0.01% Substances insoluble in dilute hydrochloric acid
≤0.2% Substances not precipitated by dilute sulfuric acid (as sulfate)
pH
14 (20 °C in H2O, saturated aqueous solution)
Punto di fusione
78 °C
Solubilità
72 g/L
Densità
2.18 g/cm3 at 20 °C
Densità bulk
900‑1100 kg/m3
Anioni in tracce
carbonate (as BaCO3): ≤2.0%
chloride (Cl-): ≤0.002%
sulfide (S2-): ≤0.001%
Cationi in tracce
Ca: ≤0.005%
Fe: ≤0.001%
Sr: ≤1.0%
heavy metals (as Pb): ≤0.002%
Temperatura di conservazione
2-30°C
Stringa SMILE
[Ba+2].[O-H].[O-H].O.O.O.O.O.O.O.O
InChI
1S/Ba.10H2O/h;10*1H2/q+2;;;;;;;;;;/p-2
ZUDYPQRUOYEARG-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Chitosan and carbon nitride doped barium hydroxide nanoparticles served as dye degrader and bactericidal potential: A molecular docking study.: This study explores the use of barium hydroxide octahydrate nanoparticles doped with chitosan and carbon nitride for applications in dye degradation and as bactericidal agents. The research highlights the potential of these nanoparticles in environmental remediation and antimicrobial treatments (Ikram et al., 2023).
- Ba(OH)2 Equilibria in the System Ba-O-H-F, With Application to the Formation of Ba2YCu3O6.5 + x From BaF2-Precursors.: This paper discusses the equilibrium of barium hydroxide octahydrate in the Ba-O-H-F system and its application in forming Ba2YCu3O6.5 + x from BaF2 precursors. This research is relevant to material synthesis and applications in superconductors (Cook et al., 2005).
- The Effect of Nanometer Oxide Additive on the Heat-Conducting Property of Barium Hydroxide Octahydrate.: This article investigates how nanometer-scale oxide additives affect the heat-conducting properties of barium hydroxide octahydrate, highlighting potential applications in thermal management systems and heat storage (Xu et al., 2015).
- Application of capillary isotachophoresis to analysis of serum protein.: This study utilizes barium hydroxide octahydrate in the capillary isotachophoresis analysis of serum proteins, demonstrating its application in biochemical analysis and clinical diagnostics (Ono et al., 1990).
- Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium.: This research applies barium hydroxide octahydrate solutions in staining tissue sections for electron microscopy, providing insights into its use in histological and cytological studies (WATSON, 1958).
Risultati analitici
Substances insoluble in dilute hydrochloric acid: ≤ 0.01 %
Carbonate (as BaCO₃): ≤ 2.0 %
Chloride (Cl): ≤ 0.002 %
Sulphide (S): ≤ 0.001 %
Heavy metals (as Pb): ≤ 0.002 %
Ca (Calcium): ≤ 0.005 %
Fe (Iron): ≤ 0.001 %
Sr (Strontium): ≤ 1.0 %
Substances not precipitated by dilute sulfuric acid (as sulfate): ≤ 0.2 %
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Codice della classe di stoccaggio
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.