V3700
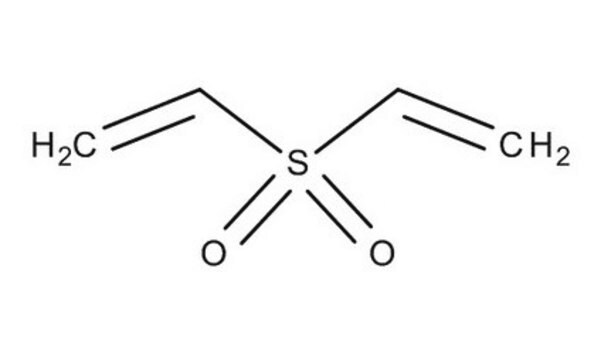
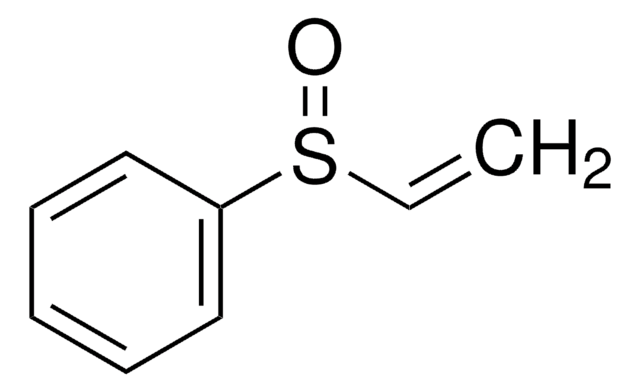
Divinyl sulfone
contains hydroquinone as inhibitor, ≥96%
Sinonimo/i:
Vinyl sulfone
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥96%
contiene
hydroquinone as inhibitor
Indice di rifrazione
n20/D 1.476 (lit.)
P. ebollizione
234 °C (lit.)
Punto di fusione
−26 °C (lit.)
Densità
1.177 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
C=CS(=O)(=O)C=C
InChI
1S/C4H6O2S/c1-3-7(5,6)4-2/h3-4H,1-2H2
AFOSIXZFDONLBT-UHFFFAOYSA-N
Informazioni sul gene
human ... LOC129293(129293)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- A cross-linking agent to synthesize divinyl sulfone-crosslinked hyaluronic acid hydrogels for specific biomedical applications, such as tissue engineering or drug delivery.
- A cross-linking agent to develop the conducting polymer film with MXene layers. This crosslinking can enhance the mechanical properties and stability of the composite film.
DVS and its mono and di-substituted derivatives are useful starting materials in the preparation of thiomorpholine 1,1-dioxides and other synthetically important macro- and the heterocycles.
DVS may be used to shrink proofing cotton by crosslinking it with cellulose.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
215.6 °F - closed cup
Punto d’infiammabilità (°C)
102 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.