H55405
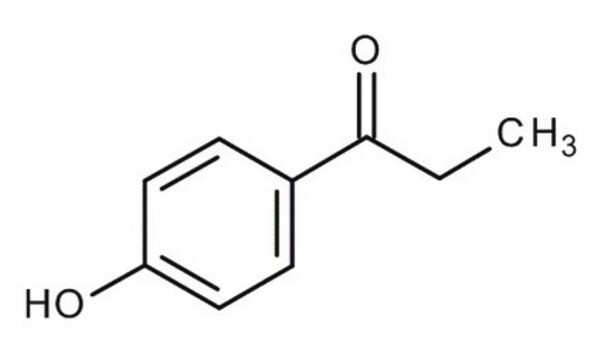
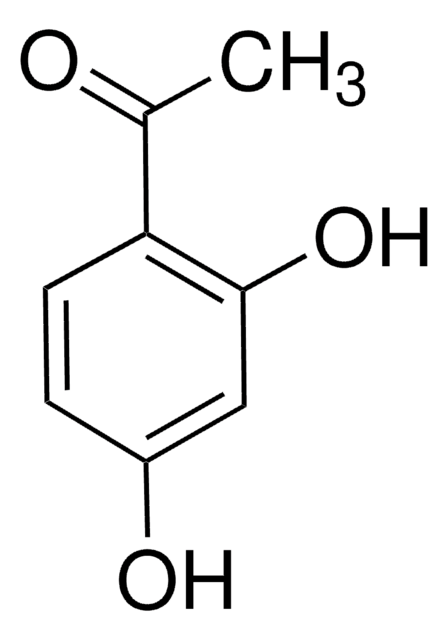
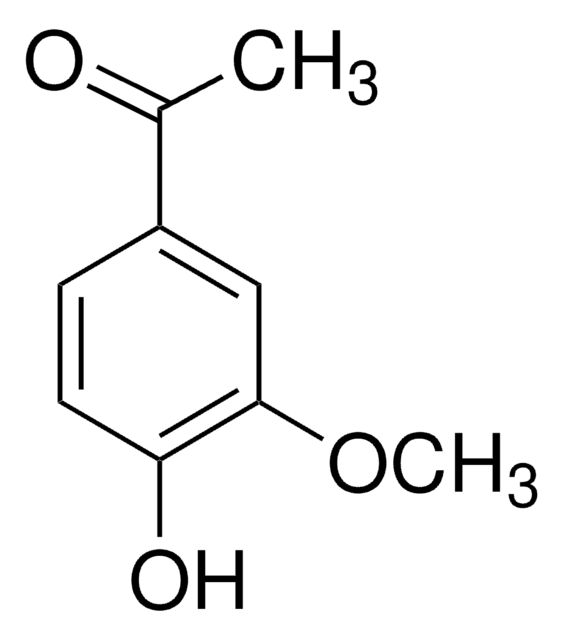
4′-Hydroxypropiophenone
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H4COC2H5
Numero CAS:
Peso molecolare:
150.17
Beilstein:
907511
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
147.5-148.5 °C (lit.)
Stringa SMILE
CCC(=O)c1ccc(O)cc1
InChI
1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
RARSHUDCJQSEFJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
p-Hydroxypropiophenone effects on azo dye-induced alterations in mouse hepatic cells: light and electron microscopic study.
N J Unakar
Journal of the National Cancer Institute, 44(4), 873-891 (1970-04-01)
A Tanner et al.
Journal of bacteriology, 182(23), 6565-6569 (2000-11-14)
An arylketone monooxygenase was purified from Pseudomonas putida JD1 by ion exchange and affinity chromatography. It had the characteristics of a Baeyer-Villiger-type monooxygenase and converted its substrate, 4-hydroxyacetophenone, into 4-hydroxyphenyl acetate with the consumption of one molecule of oxygen and
Zack E Bryant et al.
Bioorganic & medicinal chemistry letters, 21(3), 912-915 (2011-01-14)
A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, Hs578Ts(i)8 as well as of human prostate cancer cells, C4-2B. These cell
R Cizmáriková et al.
Ceskoslovenska farmacie, 42(2), 82-85 (1993-04-01)
Within the relationship of the structure and effect of new beta-adrenolytic agents derivatived from p-hydroxyacetophenone and p-hydroxypropiophenone with a propoxymethyl group in the lipophilic part of the molecule and with a propanamine, a butanamine and a pyrrolidine in the side-chain
R Cizmáriková et al.
Ceska a Slovenska farmacie : casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti, 43(5), 226-228 (1994-10-01)
The present paper carries out the pharmacological evaluation of 4-(2-hydroxy-3-isopropylaminopropoxy)-3-(alkoxymethyl) propiophenones with an ethoxy, propoxy and butoxy-group, whose structures are typical of the blockers of beta-adrenergic receptors. In the above-mentioned compounds the anticalcium effect on the frequency and the amplitude
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.