120618
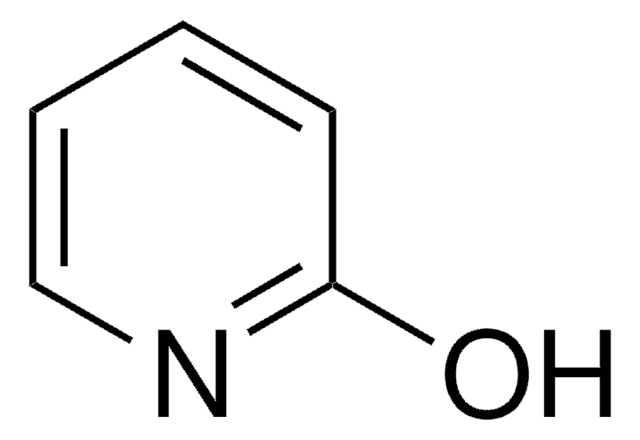
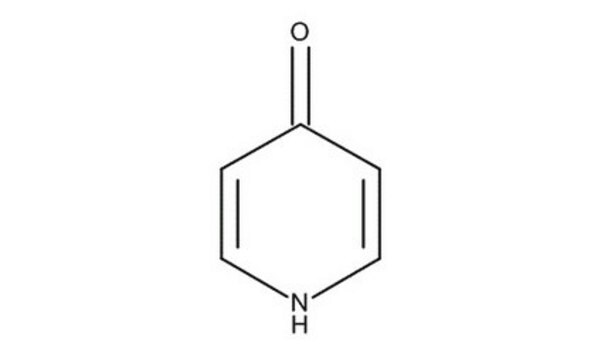
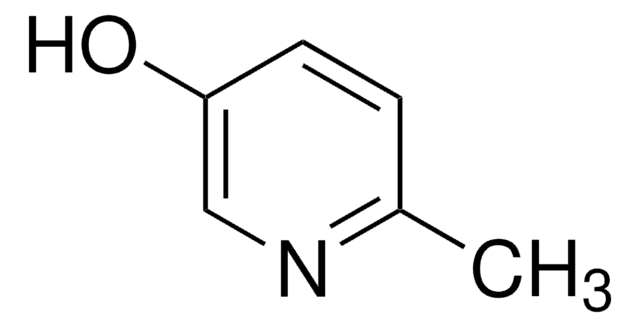
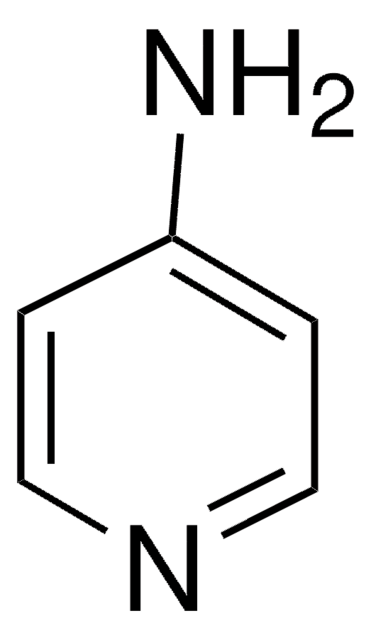
4-Hydroxypyridine
95%
Sinonimo/i:
4-Pyridinol, 4-Pyridone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H5NO
Numero CAS:
Peso molecolare:
95.10
Beilstein:
105800
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
P. ebollizione
230-235 °C/12 mmHg (lit.)
Punto di fusione
150-151 °C (lit.)
Stringa SMILE
O=C1C=CNC=C1
InChI
1S/C5H5NO/c7-5-1-3-6-4-2-5/h1-4H,(H,6,7)
GCNTZFIIOFTKIY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
4-Hydroxypyridine was used in the synthesis of (Ag3MoO3F3) (Ag3MoO4)Cl by hydro(solvato)thermal methods. It was used as model compound to study the natural photodegradation of representative aquatic environmental contaminants.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Natsuki K Kubota et al.
Bioorganic & medicinal chemistry, 11(21), 4569-4575 (2003-10-07)
Piericidins C5 (1) and C6 (2), two new members of the piericidin family, were isolated from a Streptomyces sp. and a Nocardioides sp., together with known piericidins C1 (3), C2 (4), C3 (5), C4 (6), D1 (7), and A3 (8).
Xiaoqing Cai et al.
Bioorganic & medicinal chemistry, 20(11), 3584-3595 (2012-05-09)
Bicyclic pyridinol antioxidants have been reported to suppress the autoxidation of methyl linoleate more effectively than α-tocopherol in benzene solution. A few novel lipophilic analogues have recently been synthesized by conjugating a pyridinol core with the phytyl side chain of
Alignment of acentric MoO3F33-anions in a polar material :(AgMoO3F3)(Ag3MoO4) Cl.
Maggard PA, et al.
Journal of Solid State Chemistry, 175(1), 27-33 (2003)
Heiko Zettl et al.
ACS chemical biology, 7(9), 1488-1495 (2012-06-26)
We present an integrated approach to identify and optimize a novel class of γ-secretase modulators (GSMs) with a unique pharmacological profile. Our strategy included (i) virtual screening through application of a recently developed protocol (PhAST), (ii) synthetic chemistry to discover
H Chen et al.
Biochemistry, 32(43), 11591-11599 (1993-11-02)
We have examined the interaction of Citrobacter freundii tyrosine phenol-lyase with both L- and D-alanine. This enzyme catalyzes the racemization of alanine as a side reaction, in addition to the physiological beta-elimination of L-tyrosine to give phenol and ammonium pyruvate.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.