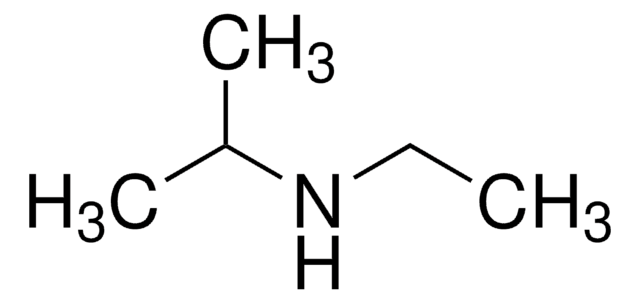
D98203
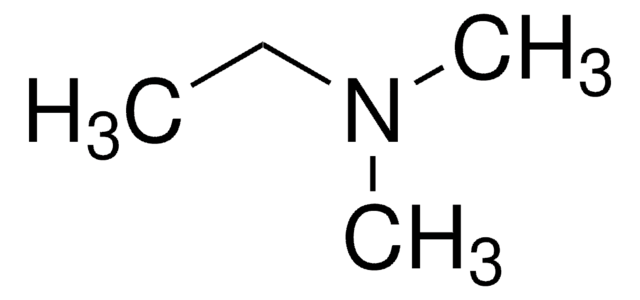
N,N-Diethylmethylamine
97%
Sinonimo/i:
N-Methyldiethylamine
About This Item
Prodotti consigliati
Saggio
97%
Stato
liquid
Indice di rifrazione
n20/D 1.389 (lit.)
P. ebollizione
63-65 °C (lit.)
Densità
0.72 g/mL at 25 °C (lit.)
Stringa SMILE
CCN(C)CC
InChI
1S/C5H13N/c1-4-6(3)5-2/h4-5H2,1-3H3
GNVRJGIVDSQCOP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- N,N-Diethylmethylamine as lineshape standard for NMR above 130 K.: This study explores the use of N,N-Diethylmethylamine as a lineshape standard for nuclear magnetic resonance (NMR) spectroscopy at temperatures above 130 K, providing insights into its applications in high-precision NMR analysis (Fritzsching et al., 2018).
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
-11.2 °F - closed cup
Punto d’infiammabilità (°C)
-24 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.