C99000
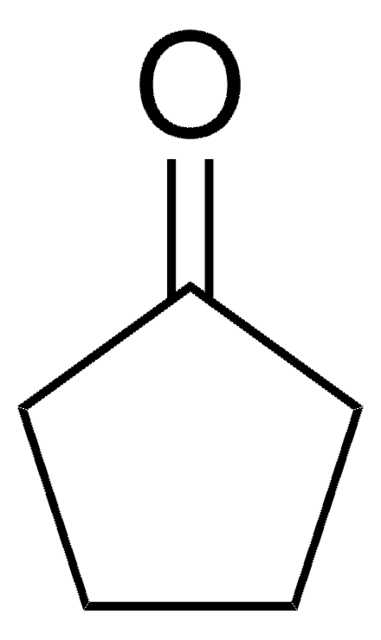
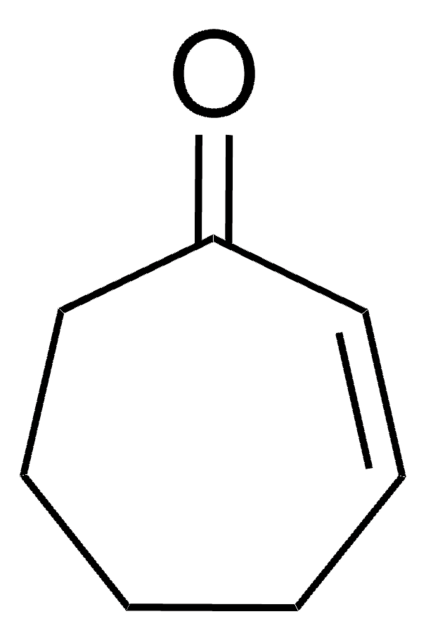
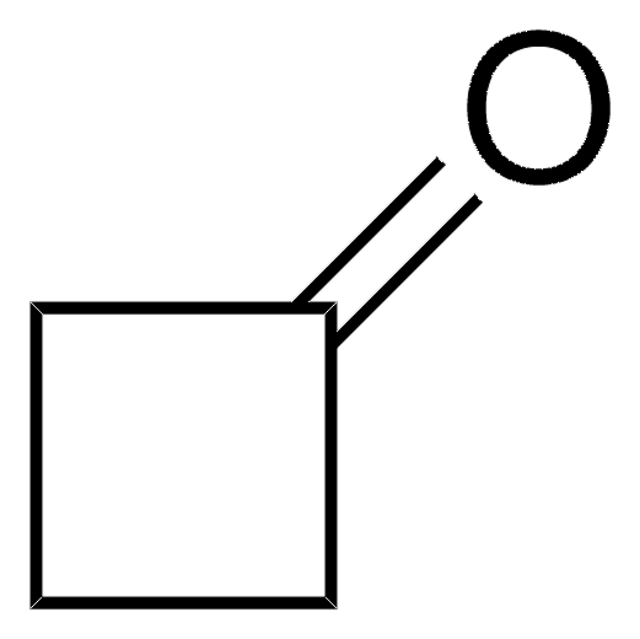
Cycloheptanone
99%
Sinonimo/i:
Ketocycloheptane, Ketoheptamethylene, Suberone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C7H12(=O)
Numero CAS:
Peso molecolare:
112.17
Beilstein:
969823
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
liquid
Indice di rifrazione
n20/D 1.461 (lit.)
P. ebollizione
179 °C (lit.)
Densità
0.951 g/mL at 25 °C (lit.)
Stringa SMILE
O=C1CCCCCC1
InChI
1S/C7H12O/c8-7-5-3-1-2-4-6-7/h1-6H2
CGZZMOTZOONQIA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
131.0 °F
Punto d’infiammabilità (°C)
55 °C
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Brian T Sullivan et al.
Journal of chemical ecology, 47(1), 10-27 (2021-01-07)
We investigated geographic variation in the semiochemistry of major disturbance agents of western North American pine forests, Dendroctonus brevicomis Le Conte and Dendroctonus barberi Hopkins (Coleoptera: Curculionidae: Scolytinae), species separated by the Great Basin in the USA that until recently
R Singh et al.
Current medicinal chemistry. Anti-cancer agents, 3(6), 431-438 (2003-10-08)
A series of naturally occurring and synthetic novel oxapenam (4-oxa-1-azabicyclo[3.2.0] heptan-7-one) derivatives with their antitumor activity and the structure-activity relationship among this class of compounds is reported. Among the synthetic 4-oxa-1-azabicyclo[3.2.0]heptan-7-one having an ester, amide, ether derivatives of hydroxy group
Richmond Sarpong et al.
Journal of the American Chemical Society, 125(45), 13624-13625 (2003-11-06)
A set of mild processes for the conversion of vinyl cyclopropyl diazo ketones to highly functionalized cycloheptadienones and vinyl cyclopentenones by use of a target-inspired tandem Wolff/Cope rearrangement sequence is described. A divergent reaction course of the vinyl cyclopropyl diazo
M Juza
Journal of chromatography. A, 865(1-2), 35-49 (2000-02-16)
A binary test mixture consisting of cyclopentanone and cycloheptanone is used for the performance evaluation of a pilot-scale simulated moving bed unit. The involved adsorption equilibria and the kinetic behavior are discussed in detail. The results of the test runs
Redouane Beniazza et al.
The Journal of organic chemistry, 76(3), 791-799 (2011-01-13)
A short access to homocalystegine analogues silylated at C7 is described. The synthesis involves the desymmetrization of a (phenyldimethylsilyl)methylcycloheptatriene using osmium-mediated dihydroxylation, followed by the diol protection and a cycloaddition involving the remaining diene moiety and an acylnitroso reagent. Additions
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.