C112208
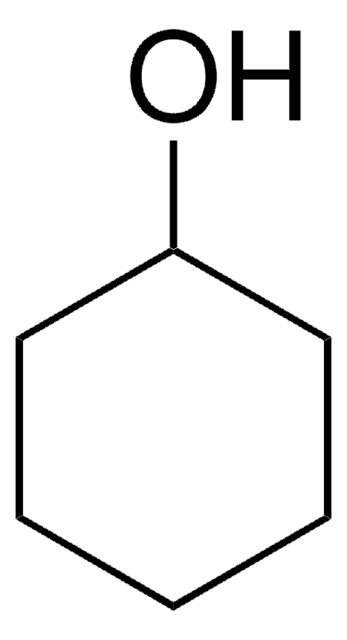
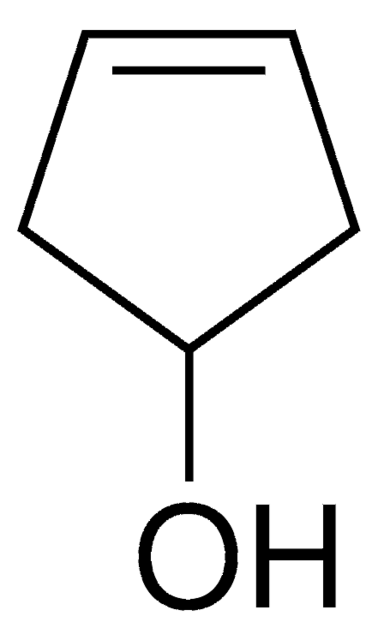
Cyclopentanol
99%
Sinonimo/i:
1-Cyclopentanol, Cyclopentyl alcohol, Hydroxycyclopentane
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
liquid
Indice di rifrazione
n20/D 1.453 (lit.)
P. ebollizione
139-140 °C (lit.)
Punto di fusione
−19 °C (lit.)
Densità
0.948 g/mL at 20 °C
0.949 g/mL at 25 °C (lit.)
Stringa SMILE
OC1CCCC1
InChI
1S/C5H10O/c6-5-3-1-2-4-5/h5-6H,1-4H2
XCIXKGXIYUWCLL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- An alkylating agent in the preparation of alkylated aromatic compounds using Fe3+-montmorillonite catalyst via Friedel–Crafts alkylation reaction.
- A reactant in the acylation of alcohols with an acid anhydride or acid chloride.
- A substrate in the synthesis of high-density polycyclic aviation fuel by the Guerbet reaction.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
123.8 °F - closed cup
Punto d’infiammabilità (°C)
51 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| C112208-100ML | 4061833460825 |
| C112208-500ML | 4061833460849 |
| C112208-5ML | 4061837637599 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.