C7206
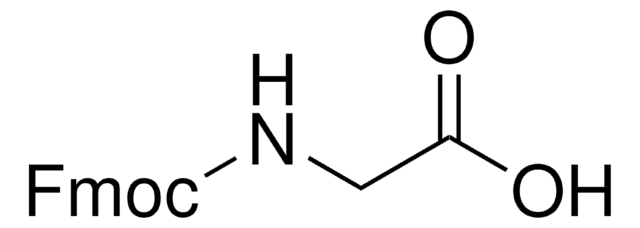
Z-Gly-OH
99%
Sinonimo/i:
Z-Glycine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5CH2OOCNHCH2COOH
Numero CAS:
Peso molecolare:
209.20
Beilstein:
526877
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
powder
Impiego in reazioni chimiche
reaction type: solution phase peptide synthesis
Colore
beige
Punto di fusione
118-122 °C (lit.)
applicazioni
peptide synthesis
Stringa SMILE
OC(=O)CNC(=O)OCc1ccccc1
InChI
1S/C10H11NO4/c12-9(13)6-11-10(14)15-7-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,14)(H,12,13)
CJUMAFVKTCBCJK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Z-Gly-OH, also known as N-benzyloxycarbonylglycine, is an amino acid widely used in solution phase peptide synthesis.
Applicazioni
Z-Gly-OH is a versatile reagent that can be used to synthesize a variety of compounds such as:
- glycine-derived peptides like Z-Gly-DL-Ala-OBzl and Z-Gly-L-Ala-OBzl
- glycine N-substituted amides such as glycine-N-methylamide hydrochloride and glycine-N-isopropylamide hydrochloride
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Studies on Separation of Amino Acids and Related Compounds. V. A Racemization Test in Peptide Synthesis by the Use of an Amino Acid Analyzer
Bulletin of the Chemical Society of Japan, 44, 3391-3395 (1971)
Duality of mechanism in the tetramethylfluoroformamidinium hexafluorophosphate-mediated synthesis of N-benzyloxycarbonylamino acid fluorides.
R Fiammengo et al.
The Journal of organic chemistry, 66(17), 5905-5910 (2001-08-21)
G K Scriba et al.
The Journal of pharmacy and pharmacology, 51(5), 549-553 (1999-07-20)
Glycine, which has weak anticonvulsant properties, has been shown to potentiate the activity of several antiepileptic drugs but not phenytoin. Recently, studies have shown that N-(benzyloxycarbonyl)glycine (Z-glycine) antagonized seizures more than glycine in addition to possessing activity in the maximal
D M Lambert et al.
Neuroreport, 5(7), 777-780 (1994-03-21)
Although glycine does not cross easily the blood-brain barrier, it exhibits at very high doses (10-40 mmol kg-1) a modest anticonvulsant activity. In this study, carbamate derivatives--N-benzyloxycarbonylglycine (Z-glycine) and N,tert-butoxycarbonylglycine (Boc-glycine)--have been compared with glycine. Z-glycine (1 mmol kg-1), but
David J Merkler et al.
Bioorganic & medicinal chemistry, 16(23), 10061-10074 (2008-10-28)
Peptidyl alpha-hydroxylating monooxygenase (PHM) functions in vivo towards the biosynthesis of alpha-amidated peptide hormones in mammals and insects. PHM is a potential target for the development of inhibitors as drugs for the treatment of human disease and as insecticides for
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.