C47604
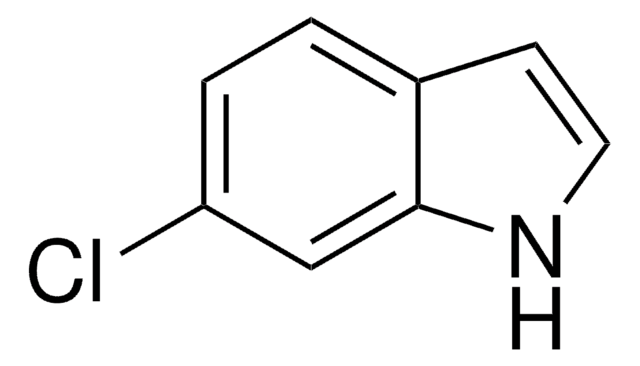
5-Chloroindole
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
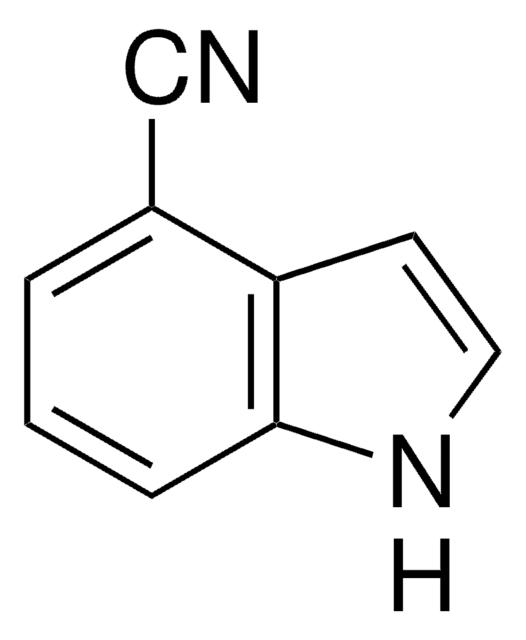
Formula empirica (notazione di Hill):
C8H6ClN
Numero CAS:
Peso molecolare:
151.59
Beilstein:
2651
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
crystals
Punto di fusione
69-71 °C (lit.)
Stringa SMILE
Clc1ccc2[nH]ccc2c1
InChI
1S/C8H6ClN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
MYTGFBZJLDLWQG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
5-Chloroindole can be synthesized by using 3-chlorobenzaldehyde as starting reagent.
5-Chloroindole is a 5-substituted indole. It undergoes electropolymerization to form a redox-active film consisting of a cyclic trimer and chains of linked cyclic trimer (polymer). It is a potential positive allosteric modulator (PAM) of the 5-HT3 receptor. It has been reported as strong inhibitor of the copper dissolution in acidic sodium chloride solution. It has been tested as corrosion inhibitor of mild steel in 1N deaerated sulphuric acid. Synthesis of 5-chloroindole, via nitration of indoline has been described.
Applicazioni
5-Chloroindole has been used in the synthesis of 5-chloro-3-indole-N,N- dimethylglyoxalamide and 5-chloro-N,N-dimethyltryptamine. It may be used in the synthesis of dyestuffs in the presence of biocatalysts (Escherichia coli expressing multicomponent phenol hydroxylase (mPH) isolated from Pseudomonas sp. strains KL33 and KL28).
5-Chloroindole has been used to study the biotransformation of substituted indoles to indican derivatives in the tissue cultures of Polygonum tinctorium. It may be employed as a monomer in the preparation of redox-active film made up of a cyclic trimer and chains of linked cyclic trimer (polymer).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Indole and 5-chloroindole as inhibitors of anodic dissolution and cathodic deposition of copper in acidic chloride solutions.
Scendo M, et al.
J. Appl. Electrochem., 33(3-4), 287-293 (2003)
Fluorescence properties of electropolymerised 5-substituted indoles in solution.
Jennings P, et al.
J. Chem. Soc., Faraday Trans., 94(24), 3619-3624 (1998)
Xiaoxue Tong et al.
Microbial cell factories, 15(1), 180-180 (2016-10-23)
Engineering of single-species biofilms for enzymatic generation of fine chemicals is attractive. We have recently demonstrated the utility of an engineered Escherichia coli biofilm as a platform for synthesis of 5-halotryptophan. E. coli PHL644, expressing a recombinant tryptophan synthase, was
Synthesis of Some 5-and 6-Chloro, 5-Methyl, and 5, 6, 7-Trimethyl Derivatives of Tryptamine.
Benington F, et al.
The Journal of Organic Chemistry, 25(9), 1542-1547 (1960)
5-Amino-and 5-chloro-indole as mild steel corrosion inhibitors in 1 N sulphuric acid.
Moretti G, et al.
Electrochimica Acta, 41(13), 1971-1980 (1996)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.