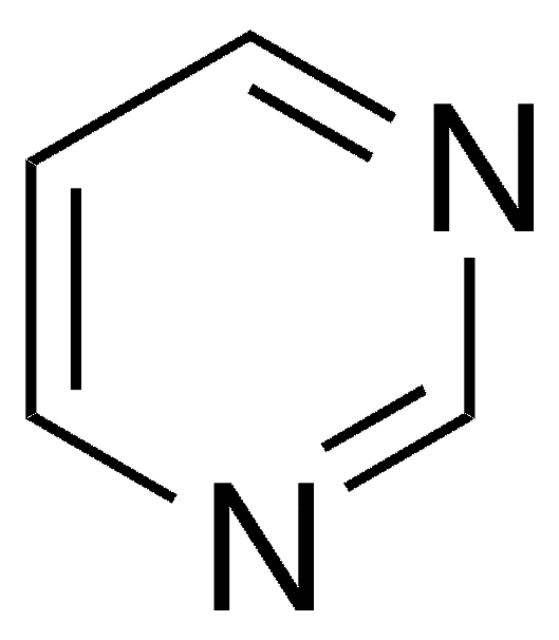
A93607
4-Azabenzimidazole
99%
Sinonimo/i:
1H-Imidazo[4,5-b]pyridine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H5N3
Numero CAS:
Peso molecolare:
119.12
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
32151902
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
powder
Punto di fusione
148-151 °C (lit.)
Stringa SMILE
c1cnc2nc[nH]c2c1
InChI
1S/C6H5N3/c1-2-5-6(7-3-1)9-4-8-5/h1-4H,(H,7,8,9)
GAMYYCRTACQSBR-UHFFFAOYSA-N
Categorie correlate
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
L Bukowski et al.
Archiv der Pharmazie, 324(2), 121-127 (1991-02-01)
New derivatives of imidazo[4,5-b]pyridine and 9H-dipyrido-[1,2-a:3',2'-d]imidazole were synthesized. Antibacterial activity against Mycobacterium tuberculosis of selected compounds was determined. These data were combined with the corresponding bioactivity data previously generated for two other series of imidazo[4,5-b]pyridines. Analysis of Quantitative Structure-Activity Relationships
S Piras et al.
Farmaco (Societa chimica italiana : 1989), 48(9), 1249-1259 (1993-09-01)
Twenty compounds possessing benzimidazole, imidazo[4,5-b]pyridine and quinoxaline structure bearing either a substituted arylmethylmercapto- or an arylmethylsulfinyl group in position 2 were prepared in order to evaluate an antiulcer and gastroprotective activity in rat pylorus ligature, in comparison with omeprazole at
Fabiola Zapata et al.
Organic letters, 10(1), 41-44 (2007-12-07)
A new redox, chromogenic, and fluorescent chemosensor molecule based on a deazapurine ring selectively senses aqueous Pb2+ in acetonitrile over other metal ions examined: redox shift (DeltaE1/2 = 0.15 V of the Fe(II)/Fe(III) redox couple), the colorless to orange color
Vassilios Bavetsias et al.
Bioorganic & medicinal chemistry letters, 17(23), 6567-6571 (2007-10-16)
A hit generation and exploration approach led to the discovery of 31 (2-(4-(6-chloro-2-(4-(dimethylamino)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)piperazin-1-yl)-N-(thiazol-2-yl)acetamide), a potent, novel inhibitor of Aurora-A, Aurora-B and Aurora-C kinases with IC(50) values of 0.042, 0.198 and 0.227microM, respectively. Compound 31 inhibits cell proliferation and has good
Ping Lan et al.
European journal of medicinal chemistry, 46(1), 77-94 (2010-11-26)
3D-QSAR and docking studies were performed on sixty imidazo[4,5-b]pyridine derivatives as Aurora A kinase inhibitors. The CoMFA and CoMSIA models using forthy-eight molecules in the training set, gave r(cv)(2) values of 0.774 and 0.800, r(2) values of 0.975 and 0.977
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![Imidazo[1,2-a]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/109/863/81ccb63f-07c6-4271-b317-1ba58979d455/640/81ccb63f-07c6-4271-b317-1ba58979d455.png)
![3-Methyl-3H-imidazo[4,5-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/309/037/0969ed2b-d1ce-49e9-87c8-ef436d41719a/640/0969ed2b-d1ce-49e9-87c8-ef436d41719a.png)


![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)