A7300
N-Acetylbenzoquinoneimine
Sinonimo/i:
N-(4-oxo-1-cyclohexa-2,5-dienylidene)acetamide, N-Acetyl-p-benzo-quinone imine, NAPQI
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H7NO2
Numero CAS:
Peso molecolare:
149.15
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Stato
solid
Livello qualitativo
Temperatura di conservazione
−70°C
Stringa SMILE
CC(=O)\N=C1\C=CC(=O)C=C1
InChI
1S/C8H7NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5H,1H3
URNSECGXFRDEDC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Reactant involved in:
- Redox reactions
- Hydrohalogenation
- pH dependent reactions: in acidic media it is hydrolyzed, hydroxylated in alkaline media, and dimerized at intermediate pHs
- Mediation of acetaminophen hepatotoxicity
- Studies to identify the utility of acetaminophen for treating autoimmune disorders
Altre note
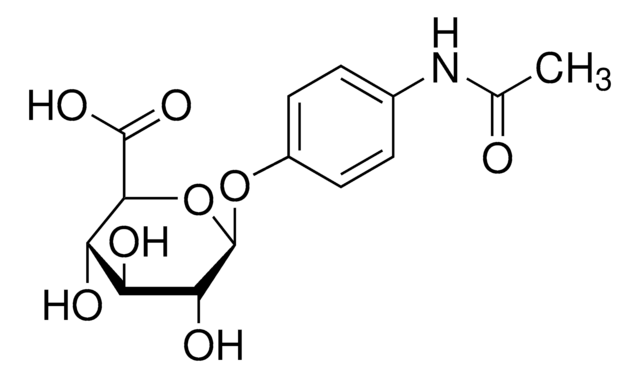
Acetaminophen metabolite that reacts with serum proteins.
Avvertenza
air, moisture and light sensitive
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Angela S Burke et al.
Chemical research in toxicology, 23(7), 1286-1292 (2010-06-29)
Acetaminophen (APAP) toxicity in primary mouse hepatocytes occurs in two phases. The initial phase (0-2 h) occurs with metabolism to N-acetyl-p-benzoquinoneimine which depletes glutathione, and covalently binds to proteins, but little toxicity is observed. Subsequent washing of hepatocytes to remove
Laura P James et al.
Drug metabolism and disposition: the biological fate of chemicals, 37(8), 1779-1784 (2009-05-15)
Acetaminophen (APAP)-induced liver toxicity occurs with formation of APAP-protein adducts. These adducts are formed by hepatic metabolism of APAP to N-acetyl-p-benzoquinone imine, which covalently binds to hepatic proteins as 3-(cystein-S-yl)-APAP adducts. Adducts are released into blood during hepatocyte lysis. We
Amaresh Kumar Sahoo et al.
Nanoscale, 3(10), 4226-4233 (2011-09-08)
Herein, we report the generation of a composite comprised of p-hydroxyacetanilide dimer and Ag nanoparticles (NPs) by reaction of AgNO(3) and p-hydroxyacetanilide. The formation of the composite was established by UV-vis, FTIR and NMR spectroscopy, transmission electron microscopy and X-ray
Hui Ting Chng et al.
Journal of biomolecular screening, 17(7), 974-986 (2012-05-31)
The zebrafish model has been increasingly explored as an alternative model for toxicity screening of pharmaceutical drugs. However, little is understood about the bioactivation of drug to reactive metabolite and phase I and II metabolism of chemical in zebrafish as
Cross-linking of protein molecules by the reactive metabolite of acetaminophen, N-acetyl-p-benzoquinone imine, and related quinoid compounds.
A J Streeter et al.
Advances in experimental medicine and biology, 197, 727-737 (1986-01-01)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.