804088
SnAP 2-Spiro-(4-Pip) M Reagent
Sinonimo/i:
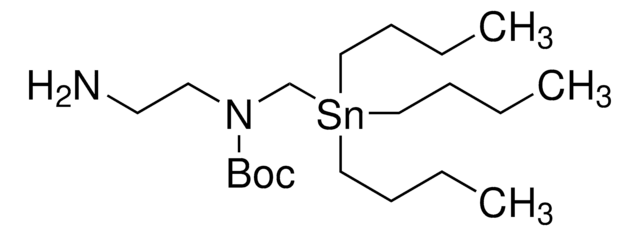
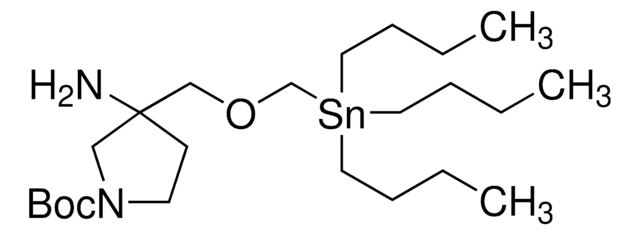
tert-butyl 4-(aminomethyl)-4-((tributylstannyl)methoxy)piperidine-1-carboxylate
About This Item
Prodotti consigliati
Stato
liquid
Indice di rifrazione
n/D 1.492
Densità
1.146
Gruppo funzionale
amine
ether
Temperatura di conservazione
−20°C
Stringa SMILE
CCCC[Sn](CCCC)(COC1(CCN(C(OC(C)(C)C)=O)CC1)CN)CCCC
InChI
1S/C12H23N2O3.3C4H9.Sn/c1-11(2,3)17-10(15)14-7-5-12(9-13,16-4)6-8-14;3*1-3-4-2;/h4-9,13H2,1-3H3;3*1,3-4H2,2H3;
LEBKPKYDLQUOJO-UHFFFAOYSA-N
Descrizione generale
Applicazioni
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
Altre note
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
Bespoke SnAP Reagents for the Synthesis of C-Substituted Spirocyclic and Bicyclic Saturated N-Heterocycles
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
>230.0 °F
Punto d’infiammabilità (°C)
> 110 °C
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Protocolli
Saturated N-heterocyclic building blocks or SnAP Reagents are of growing importance for the convenient synthesis of medium-ring saturated N-heterocycles, including bicyclic and spirocyclic structures. SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.