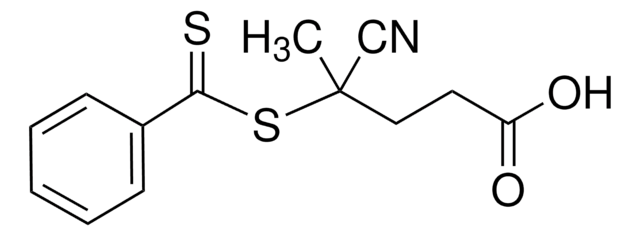
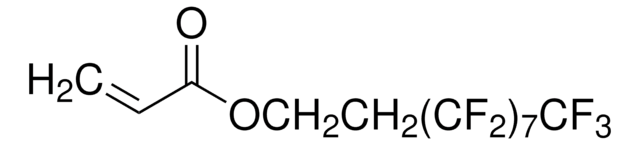741108
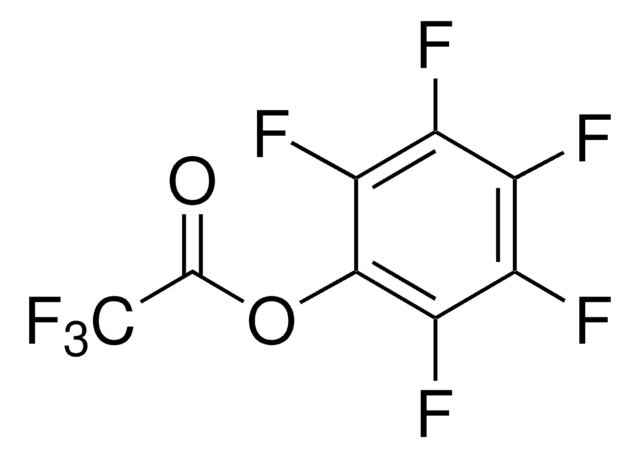
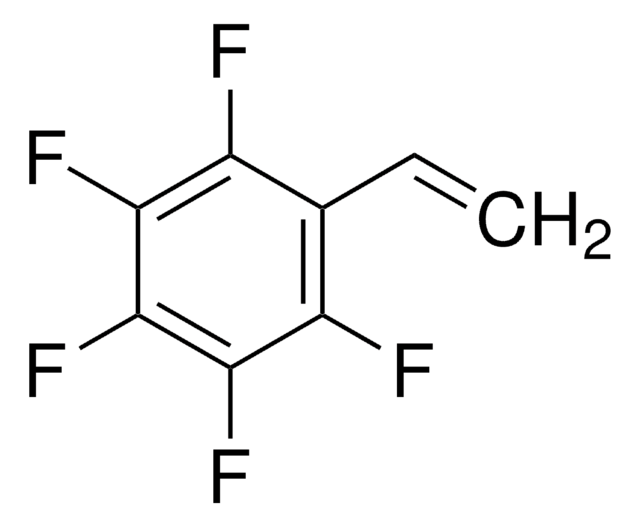
Pentafluorophenyl methacrylate
contains MEHQ as inhibitor, 95%
Sinonimo/i:
Methacrylic acid, pentafluorophenyl ester
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H5F5O2
Numero CAS:
Peso molecolare:
252.14
Numero MDL:
Codice UNSPSC:
12162002
ID PubChem:
NACRES:
NA.23
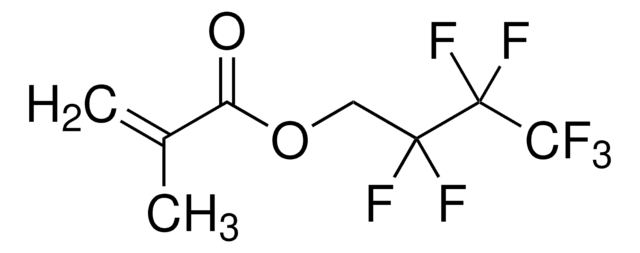
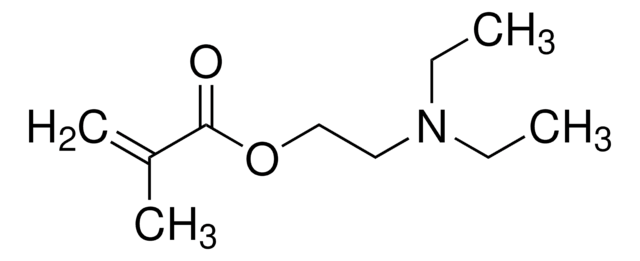
Prodotti consigliati
Saggio
95%
Forma fisica
liquid
contiene
MEHQ as inhibitor
Indice di rifrazione
n20/D 1.438
Densità
1.394 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(=C)C(=O)Oc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C10H5F5O2/c1-3(2)10(16)17-9-7(14)5(12)4(11)6(13)8(9)15/h1H2,2H3
NIJWSVFNELSKMF-UHFFFAOYSA-N
Categorie correlate
Applicazioni
The PFP unit acts as an activated ester for coupling (esterification/amidation) reactions. This is also a monomer for low refractive index polymers (n ~1.40).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
172.0 °F
Punto d’infiammabilità (°C)
77.8 °C
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
[Copolymerization of methyl methacrylate with pentafluorophenyl methacrylate and physical characteristics of the polymers (author's transl)].
Y Kadoma et al.
Iyo Kizai Kenkyujo hokoku. Reports of the Institute for Medical and Dental Engineering, Tokyo Medical and Dental University, 15, 23-29 (1981-01-01)
Luis Duque et al.
Biomacromolecules, 11(10), 2818-2823 (2010-09-14)
Thin films of plasma polymerized pentafluorophenyl methacrylate (pp-PFM) offer highly reactive ester groups throughout the structure of the film that allow for subsequent reactions with different aminated reagents and biological molecules. The present paper follows on from previous work on
Claudia Battistella et al.
Biomacromolecules, 18(6), 1855-1865 (2017-04-15)
Inhibition of P-glycoprotein (P-gp) transporter is an attractive approach for the reversion of cancer-associated multidrug resistance (MDR). Poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA)-based carriers that are able to release the anticancer drug doxorubicin in the lysosomes have shown promise to reduce P-gp mediated
Nikhil K Singha et al.
Biomacromolecules, 12(8), 2908-2913 (2011-07-08)
Herein the concept of tandem postpolymerization modification as a versatile route to synthesize well-defined, highly functionalized polymers is introduced. Poly(pentafluorophenyl methacrylate) obtained by atom transfer radical polymerization was first modified with allylamine, which displaces the active ester to give well-defined
L Francesch et al.
Langmuir : the ACS journal of surfaces and colloids, 23(7), 3927-3931 (2007-03-07)
Pulsed-plasma polymerization has been used to deposit ultrathin layers of pentafluorophenyl methacrylate by using low duty cycles and low power input. The monomer structure can be retained such that the chemical reactivity of the active ester group could be studied
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)