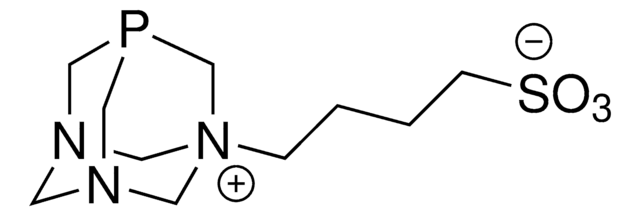
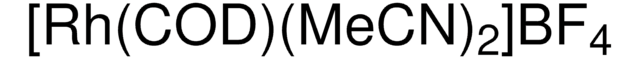695467
1,3,5-Triaza-7-phosphatricyclo[3.3.1.13,7]decane
97%
Sinonimo/i:
1,3,5-Triaza-7-phosphaadamantane, NSC 266642, PTA
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Impiego in reazioni chimiche
reagent type: ligand
reaction type: Hydroformylations
reagent type: ligand
reaction type: Hydrogenations
reagent type: ligand
reaction type: Morita-Baylis-Hillman Reactions
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
Punto di fusione
244-250 °C
Gruppo funzionale
phosphine
Stringa SMILE
C1N2CN3CN1CP(C2)C3
InChI
1S/C6H12N3P/c1-7-2-9-3-8(1)5-10(4-7)6-9/h1-6H2
FXXRPTKTLVHPAR-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- The molecular mechanisms of antimetastatic ruthenium compounds explored through DIGE proteomics.: This study examines the antimetastatic properties of ruthenium compounds using DIGE proteomics. The involvement of 1,3,5-Triaza-7-phosphaadamantane in the complexation with ruthenium and its biological effects were analyzed, highlighting its potential in anticancer therapies (Guidi et al., 2013).
- Synthesis, antimicrobial and antiproliferative activity of novel silver(I) tris(pyrazolyl)methanesulfonate and 1,3,5-triaza-7-phosphadamantane complexes.: This research details the synthesis of novel silver complexes containing 1,3,5-Triaza-7-phosphaadamantane, evaluating their antimicrobial and antiproliferative activities, which demonstrates the compound′s utility in biomedical applications (Pettinari et al., 2011).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
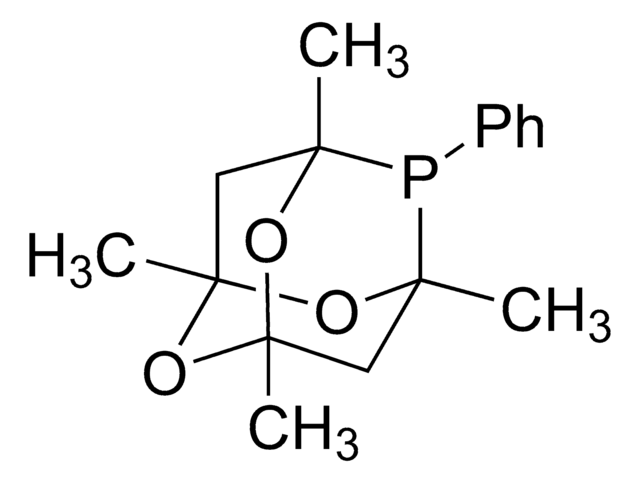
I clienti hanno visto anche
Articoli
The use of amines and phosphines in nucleophilic catalysis is well precedented; however, arguably one of the severe limitations with respect to exploiting the more nucleophilic, yet less basic, phosphine in this regard is its air sensitivity.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 695467-2G | 4061833550823 |
| 695467-100MG | |
| 695467-500MG | 4061833541098 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.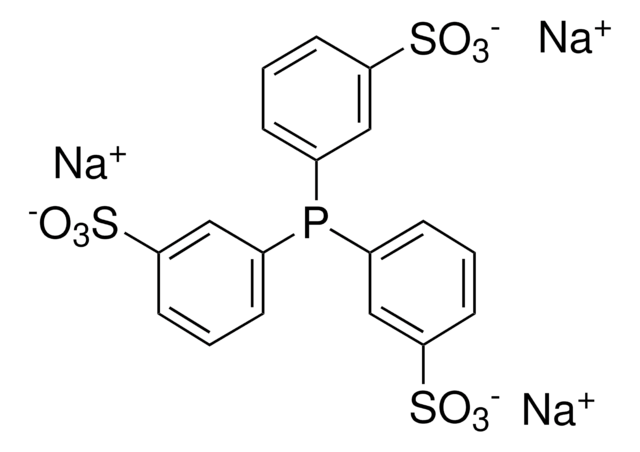
![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)
![1,4-Bis[(phenyl-3-propanesulfonate) phosphine] butane disodium salt](/deepweb/assets/sigmaaldrich/product/structures/322/102/cc3c448f-049a-41a4-93c3-26d70302d06d/640/cc3c448f-049a-41a4-93c3-26d70302d06d.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)