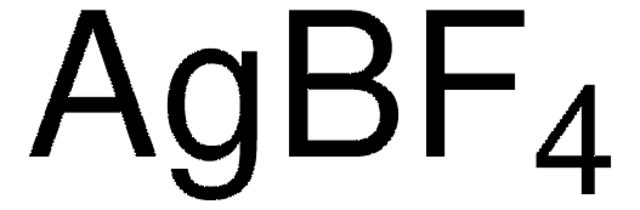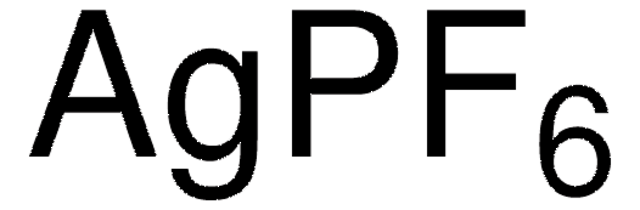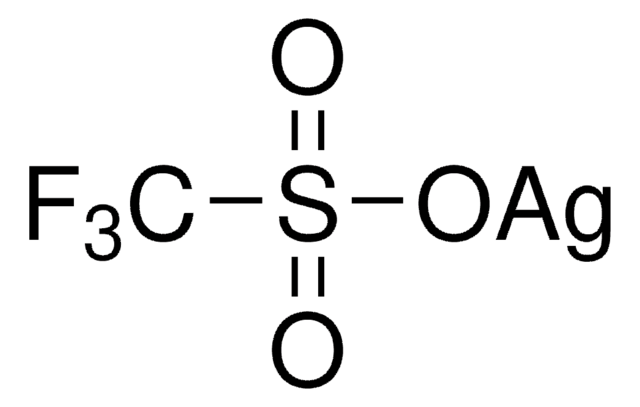674583
Silver perchlorate
anhydrous, 97%
Sinonimo/i:
Perchloric acid silver(1+) salt, Silver(1+) perchlorate
About This Item
Prodotti consigliati
Grado
anhydrous
Livello qualitativo
Saggio
97%
Stato
solid
Impiego in reazioni chimiche
reagent type: catalyst
core: silver
Impurezze
≤0.1% Insoluble matter
Punto di fusione
486 °C (lit.)
Solubilità
benzene: slightly soluble(lit.)
pyridine: slightly soluble(lit.)
Densità
2.8 g/mL at 25 °C (lit.)
Stringa SMILE
[Ag+].[O-]Cl(=O)(=O)=O
InChI
1S/Ag.ClHO4/c;2-1(3,4)5/h;(H,2,3,4,5)/q+1;/p-1
YDHABVNRCBNRNZ-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Anion exchange dynamics in the capture of perchlorate by a cationic Ag-based MOF: Investigates the anion exchange dynamics and structural transformations of a silver-based metal-organic framework capturing perchlorate (Colinas et al., 2017).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Ox. Sol. 2 - Skin Corr. 1B
Codice della classe di stoccaggio
5.1A - Strongly oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.