516112
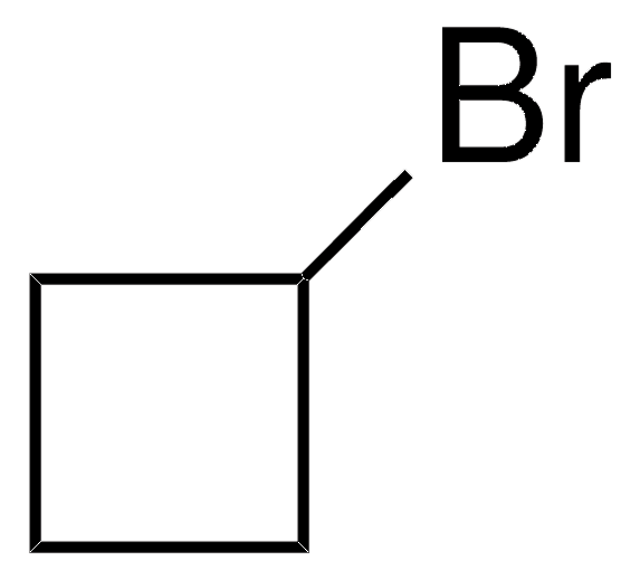
Cyclopropylacetonitrile
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
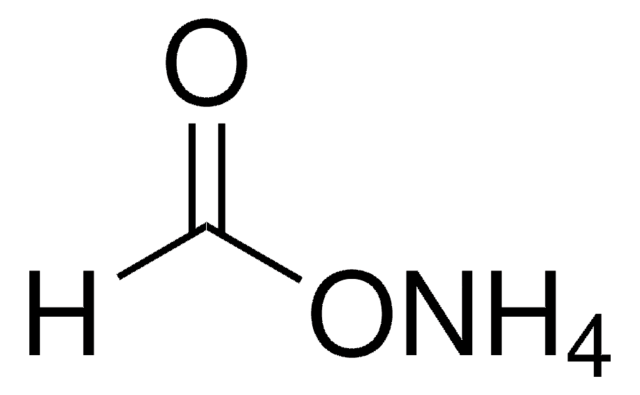
Formula condensata:
C3H5CH2CN
Numero CAS:
Peso molecolare:
81.12
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Indice di rifrazione
n20/D 1.4235 (lit.)
P. ebollizione
142-144 °C (lit.)
Densità
0.878 g/mL at 25 °C (lit.)
Gruppo funzionale
nitrile
Stringa SMILE
N#CCC1CC1
InChI
1S/C5H7N/c6-4-3-5-1-2-5/h5H,1-3H2
FAUQRRGKJKMEIW-UHFFFAOYSA-N
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
111.0 °F - closed cup
Punto d’infiammabilità (°C)
43.89 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
The Synthesis of Methyl Cyclopropylacetate by Palladium (II) Acetate Catalysed Reaction Of Diazomethane with Vinyl Group.
Basnak I, et al.
Synthetic Communications, 22(5), 773-782 (1992)
Jonathan D Rosen et al.
Tetrahedron letters, 50(7), 785-789 (2010-02-18)
An optimized total synthesis of the 2-amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core structure of a new fluoroquinolone-like class of antibacterial agents is described. This synthesis is highlighted by a nearly quantitative ring-closing reaction to form the pyrido[1,2-c]pyrimidine core. This bicyclic ring system serves as
Charles E Mowbray et al.
Journal of medicinal chemistry, 58(24), 9615-9624 (2015-11-17)
Visceral leishmaniasis is a severe parasitic disease that is one of the most neglected tropical diseases. Treatment options are limited, and there is an urgent need for new therapeutic agents. Following an HTS campaign and hit optimization, a novel series
Phyllis A Leber et al.
Molecules (Basel, Switzerland), 19(2), 1527-1543 (2014-01-30)
The title compound 1-exo (with minor amounts of its C8 epimer 1-endo) was prepared by Wolff-Kishner reduction of the cycloadduct of 1,3-cyclohexadiene and cyclopropylketene. The [1,3]-migration product 2-endo was synthesized by efficient selective cyclopropanation of endo-5-vinylbicyclo[2.2.2]oct-2-ene at the exocyclic π-bond.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.