447714
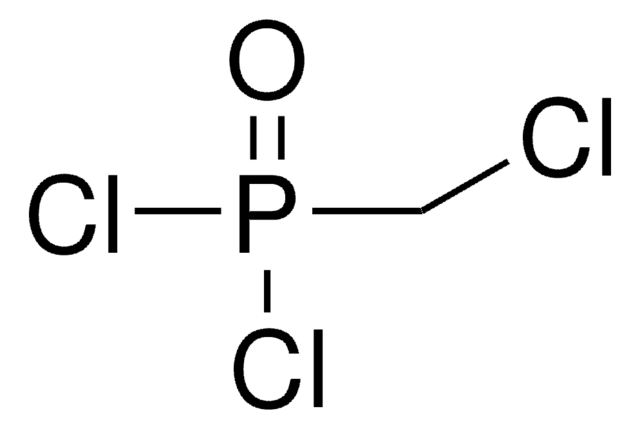
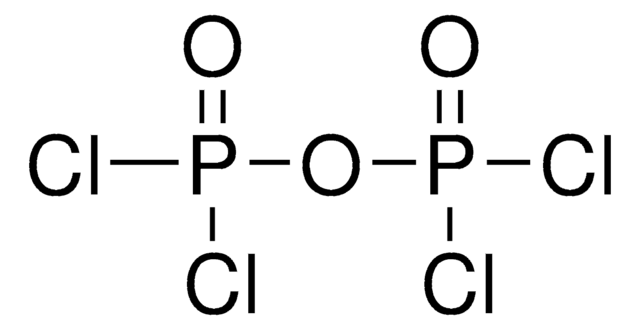
Methylenebis(phosphonic dichloride)
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
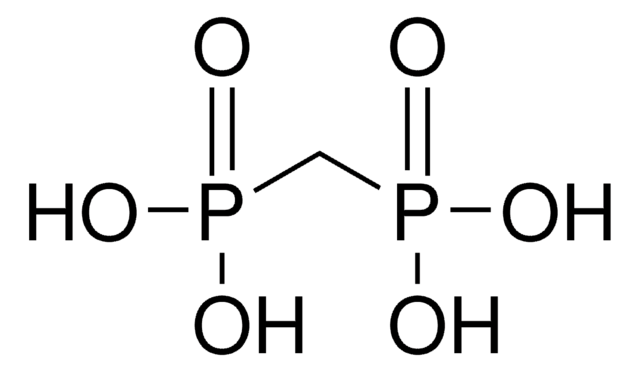
CH2[P(O)Cl2]2
Numero CAS:
Peso molecolare:
249.78
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
Punto di fusione
102-104 °C (lit.)
Gruppo funzionale
phosphine oxide
Stringa SMILE
ClP(Cl)(=O)CP(Cl)(Cl)=O
InChI
1S/CH2Cl4O2P2/c2-8(3,6)1-9(4,5)7/h1H2
VRXYCDTWIOCJBH-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Methylenebis(phosphonic dichloride) is an organophosphorus compound that is commonly used in phosphonylation reactions. It is more reactive and the rate of reaction is faster compared to POCl3. This is because the phosphorus center is more electrophilic due to the lack of electron back-donation from the CH2 group.
Applicazioni
Methylenebis(phosphonic dichloride) may be used for the following studies:
- Synthesis of mycophenolic methylenebis(phosphonate) derivatives.
- Phosphonylation of nucleosides.
- Preparation of P,P′-partial esters of methylenebisphosphonic acid.
- Synthesis of symmetrical di- and tetra- esters of methylenebisphosphonic acid.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Rischi supp
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Krzysztof W Pankiewicz et al.
Journal of medicinal chemistry, 45(3), 703-712 (2002-01-25)
Novel mycophenolic adenine dinucleotide (MAD) analogues have been prepared as potential inhibitors of inosine monophosphate dehydrogenase (IMPDH). MAD analogues resemble nicotinamide adenine dinucleotide binding at the cofactor binding domain of IMPDH; however, they cannot participate in hydride transfer and therefore
Facile high yielding synthesis of symmetric esters of methylenebisphosphonic acid.
Stepinski DC, et al.
Tetrahedron, 57(41), 8637-8645 (2001)
A direct method for the synthesis of nucleoside 5'-methylenebis (phosphonate) s from nucleosides.
Kalek M, et al.
Tetrahedron Letters, 46(!4), 2417-2421 (2005)
Aviran Amir et al.
The Journal of organic chemistry, 78(2), 270-277 (2012-12-05)
A new transformation of methylene-bis(phosphonic dichloride) into tetrathiobisphosphonate derivatives is reported. The reaction of methylene-bis(phosphonic dichloride) with 1,2-ethanedithiol in bromoform in the presence of AlCl(3) formed methylene-bis(1,3,2-dithiaphospholane-2-sulfide), which gave rise to O,O'-diester-methylenediphosphonotetrathioate analogues 1a-k upon reaction with phenols and alkyl
Sanjay Bhattarai et al.
Journal of medicinal chemistry, 63(6), 2941-2957 (2020-02-12)
CD73 inhibitors are promising drugs for the (immuno)therapy of cancer. Here, we present the synthesis, structure-activity relationships, and cocrystal structures of novel derivatives of the competitive CD73 inhibitor α,β-methylene-ADP (AOPCP) substituted in the 2-position. Small polar or lipophilic residues increased
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.