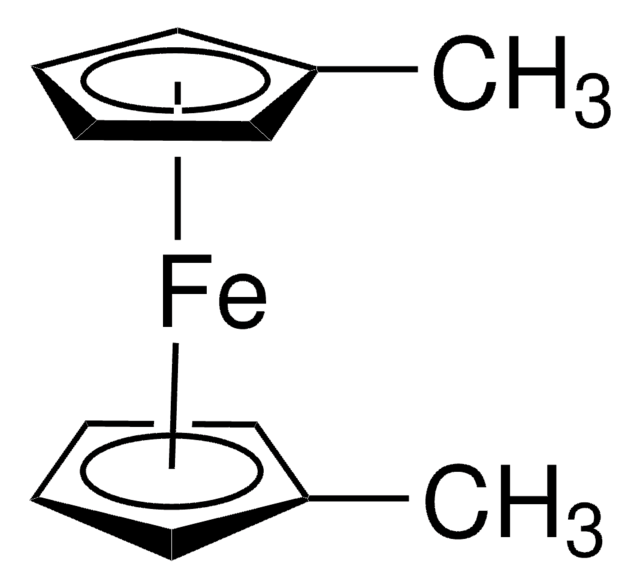
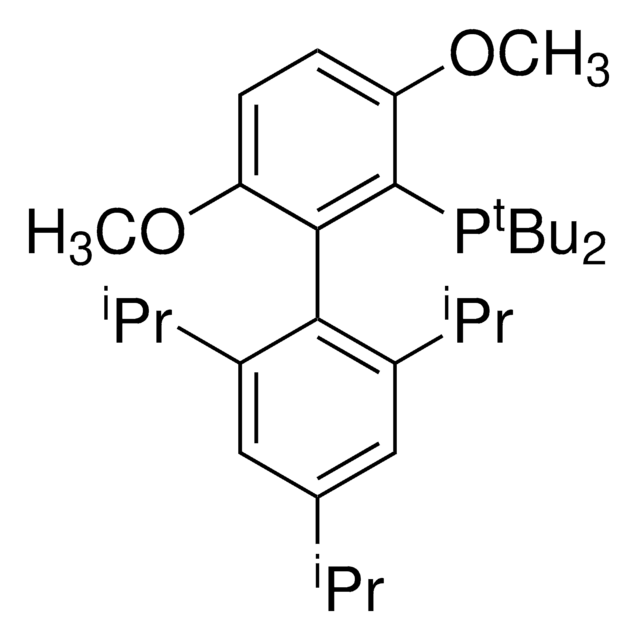
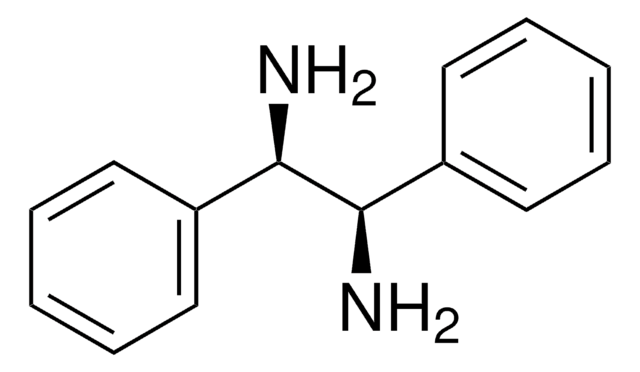
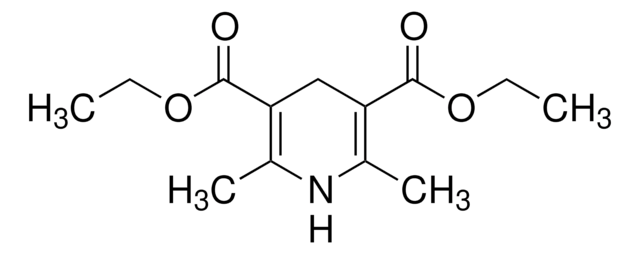
436860
N,N-Diisopropylaniline
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5N[CH(CH3)2]2
Numero CAS:
Peso molecolare:
177.29
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
liquid
Indice di rifrazione
n20/D 1.519 (lit.)
P. ebollizione
95-96 °C/11 mmHg (lit.)
Densità
0.91 g/mL at 25 °C (lit.)
Gruppo funzionale
amine
Stringa SMILE
CC(C)N(C(C)C)c1ccccc1
InChI
1S/C12H19N/c1-10(2)13(11(3)4)12-8-6-5-7-9-12/h5-11H,1-4H3
OVSARSKQWCLSJT-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
N,N-Diisopropylaniline is an N,N-dialkylaniline. Its borane adducts have been prepared and reported as hydroborating agents. Thermal dissociation of the borane-N,N-diisopropylaniline adduct has been reported to afford gaseous diborane. N,N-Diisopropylaniline is a diisoproplyamino derivative that can be prepared by reacting bromobenzene with diisopropylamine.
Applicazioni
N,N-Diisopropylaniline was used for the synthesis of 4-diisopropylamino benzonitrile. It may be used for the synthesis of 6-(4-bromophenyl)-3-methoxy-5-methyl-8-oxabicyclo[3.2.1]octa-3,6-dien-2-one and N,N,N′,N′-tetraisopropylbenzidine.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
183.2 °F - closed cup
Punto d’infiammabilità (°C)
84 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yvonne D Williams et al.
The Journal of organic chemistry, 78(23), 11707-11713 (2013-11-01)
Methoxytropolones are useful scaffolds for therapeutic development because of their known biological activity and established value in the synthesis of α-hydroxytropolones. Upon treatment with triflic acid, a series of 3-methoxy-8-oxabicyclo[3.2.1]octa-3,6-dien-2-ones rearrange rapidly and cleanly to form methoxytropolones. Interestingly, bicycles that
N-alkylation of hindered secondary aromatic amines with 2-iodobutane.
Katritzky AR, et al.
ORL; Journal for Oto-Rhino-Laryngology and Its Related Specialties, 23(4), 399-402 (1991)
Synthesis of N,N,N',N'-tetraalkylbenzidines through oxidative coupling of N,N-dialkylarylamines induced by SbCl5.
Vitale P, et al.
ARKIVOC (Gainesville, FL, United States), 3, 36-48 (2013)
Dual fluorescence and fast intramolecular charge transfer with 4-(diisopropylamino) benzonitrile in alkane solvents.
Demeter A, et al.
Chemical Physics Letters, 323(3), 351-360 (2000)
Brown et al.
The Journal of organic chemistry, 65(15), 4655-4661 (2000-08-26)
Several N,N-diethyl-tert-alkylamines, such as N,N-diethyl-2-methyl-2-butylamine (1, t-PentNEt2), N,N-diethyl-2,3-dimethyl-2-butylamine (2, t-HexNEt2), N,N-diethyl-2,3,3-trimethyl-2-butylamine (3, t-HeptNEt2), and N,N-diethyl-1,1,3,3-tetramethylbutylamine (4, t-OctNEt2) with varying steric bulk around nitrogen (by changing the tert-alkyl group) have been prepared and examined as borane carriers. The complexing ability of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.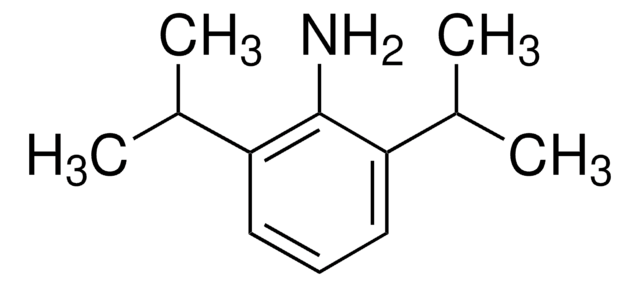
![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)