392847
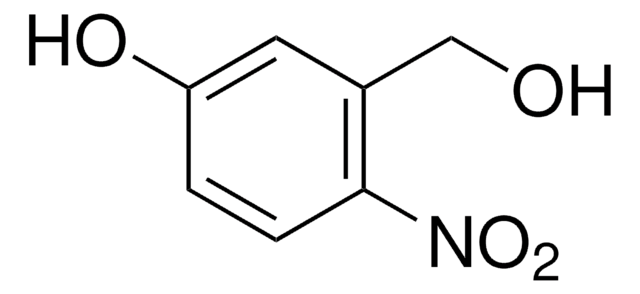
4,5-Dimethoxy-2-nitrobenzyl alcohol
98%
Sinonimo/i:
6-Nitroveratryl alcohol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
O2NC6H2(OCH3)2CH2OH
Numero CAS:
Peso molecolare:
213.19
Beilstein:
1880093
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
145-148 °C (lit.)
Gruppo funzionale
hydroxyl
nitro
Stringa SMILE
COc1cc(CO)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H11NO5/c1-14-8-3-6(5-11)7(10(12)13)4-9(8)15-2/h3-4,11H,5H2,1-2H3
WBSCOJBVYHQOFB-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-Nitroveratryl Alcohol) is 2-nitrobenzyl alcohol derivative. It has been reported to be one of the oxidation products of veratryl (3,4-dimethoxybenzyl) alcohol by lignin peroxidase (isolated from Phanerochaete chrysosporium).
Applicazioni
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-nitroveratryl alcohol) is suitable reagent used in the synthesis of 4,5-dimethoxy-2-nitrobenzyl methacrylate, a photolabile monomer and 2-(4-((4-(4,5-dimethoxy-2-nitrobenzyloxy)phenyl)cyclohexylidene)methyl)phenoxy)-N,N-dimethylethanamine, a caged cyclofen-OH ligand.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
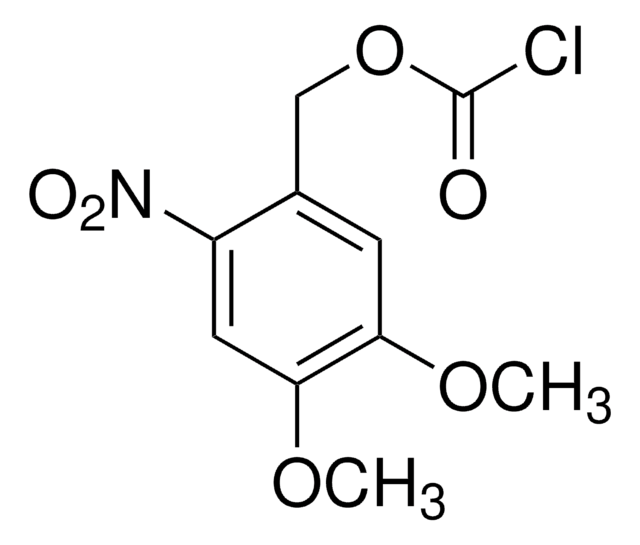
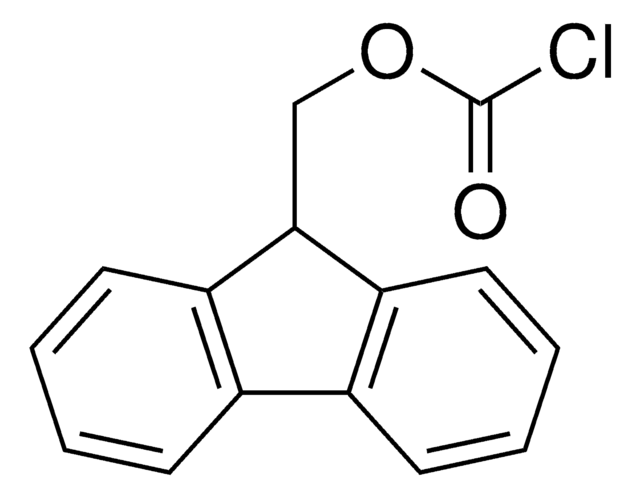
- 1-[[(chlorocarbonyl)oxy]methyl]-4,5-dimethoxy-2-nitrobenzene
- bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol, a photolabile protecting group
- optically-sensitive monomer
- nitroveratryl (NV) protected α-hydroxyacetic acid (αG) (NV-αG-OH), required in the preparation of nitroveratryl (NV) protected cyanomethyl (CM) ester of α-hydroxyacetic acid (αG) (NV-αG-CM)
- 4,5-dimethoxy-2-nitrobenzyl p-nitro-phenylcarbonate
- 6-nitroveratryloxycarbonyl chloride (NVOCCl), a reagent used in the protection of amino function in amino sugars
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Andrew A Brown et al.
Langmuir : the ACS journal of surfaces and colloids, 25(3), 1744-1749 (2009-01-10)
The use of photolabile protecting groups (PGs) as a means to create latent hydrophilic surfaces is presented. Naturally hydrophobic PGs, based on o-nitrobenzyl chemistry, are used on polymer side chains, poised for cleavage upon exposure to UV light. Removal of
Peptide backbone mutagenesis of putative gating hinges in a potassium ion channel.
Yasuo Nagaoka et al.
Chembiochem : a European journal of chemical biology, 9(11), 1725-1728 (2008-06-11)
Novel photosensitive polyimide precursor based on polyisoimide using an amine photogenerator.
Mochizuki A, et al.
Macromolecules, 28(1), 365-369 (1995)
Photosensitive protecting groups of amino sugars and their use in glycoside synthesis. 2-nitrobenzyloxycarbonylamino and 6-nitroveratryloxycarbonylamino derivatives.
Amit B, et al.
The Journal of Organic Chemistry, 39(2), 192-196 (1974)
Nadezda Fomina et al.
Journal of the American Chemical Society, 132(28), 9540-9542 (2010-06-24)
A new light-sensitive polymer containing multiple light-sensitive triggering groups along the backbone and incorporating a quinone-methide self-immolative moiety was developed and formulated into nanoparticles encapsulating a model pharmaceutical Nile Red. Triggered burst release of the payload upon irradiation and subsequent
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.