392855
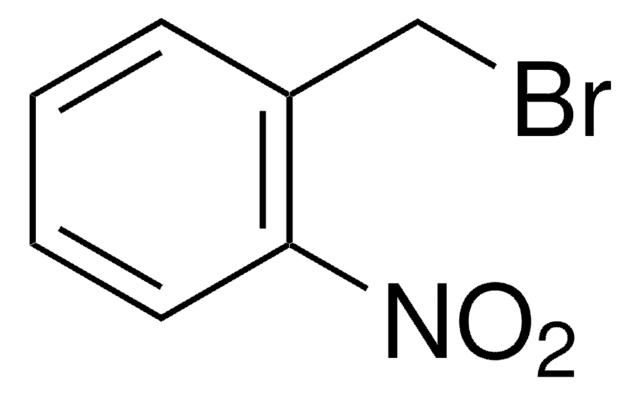
4,5-Dimethoxy-2-nitrobenzyl bromide
97%
Sinonimo/i:
6-Nitroveratryl bromide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
O2NC6H2(OCH3)2CH2Br
Numero CAS:
Peso molecolare:
276.08
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
131-133 °C (lit.)
Gruppo funzionale
bromo
nitro
Stringa SMILE
COc1cc(CBr)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H10BrNO4/c1-14-8-3-6(5-10)7(11(12)13)4-9(8)15-2/h3-4H,5H2,1-2H3
UEKFEYNZISYRRH-UHFFFAOYSA-N
Descrizione generale
4,5-Dimethoxy-2-nitrobenzyl bromide (DMNBB, 1-(Bromomethyl)-4,5-dimethoxy-2-nitrobenzene) is a 4,5-dimethoxy-2-nitrobenzyl derivative. 4,5-Dimethoxy-2-nitrobenzyl (DMNB) group of DMNBB is used as a photolabile protecting group in caging technology to develop pro-drugs. Synthesis of 1-(bromomethyl)-4,5-dimethoxy-2-nitrobenzene by using 6-nitroveratraldehyde as starting reagent has been reported.
Applicazioni
4,5-Dimethoxy-2-nitrobenzyl bromide (DMNB bromide, 6-nitroveratryl bromide) is suitable reagent used in the synthesis of N-(4,5-dimethoxy-2-nitrobenzyl)vanillylamine which forms caged vanilloid. It may be used in the synthesis of the following:
- 4-(4′,5′-dimethoxy-2-nitrobenzyloxy)benzaldehyde, a DMNB-caged aldehyde
- N-[4-[(4,5-dimethoxy-2-nitrobenzyl)oxy]-3-methoxybenzyl]acetamide
- caged derivative of pyridostatin ([C]-PDS)
- photosensitive polyimide (PI-DMNB)
- caged β-ecdysone
- 4-tert-butyldimethylsilyloxy-1-(2-deoxy-3,5-di-O-toluoyl-β-D-ribofuranosyl)-2-(6-nitroveratrylthio)-1H-benzimidazole, an intermediate in synthesis of phosphoramidite bearing 4-hydroxy-2-mercaptobenzimidazole (SBNV) nucleobase
- alkylation of dihydrofluorescein
- 4-(4′,5′-Dimethoxy-2′-nitrobenzyloxy)benzaldehyde
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Surface functionalization of PEEK films using photochemical routes.
Henneuse-Boxus C, et al.
Eur. Polymer J., 37(1), 9-18 (2001)
Pierre Murat et al.
Chemical communications (Cambridge, England), 49(76), 8453-8455 (2013-08-21)
The use of a caged G-quadruplex ligand allows for transcriptional control of quadruplex-containing genes using UV light as an external trigger. An important oncogene, SRC, involved in the initiation and proliferation of epithelial tumours is shown to be significantly downregulated
G Marriott et al.
Biochemistry, 35(10), 3170-3174 (1996-03-12)
An understanding of the molecular mechanism of muscle contraction will require a complete description of the kinetics of the myosin motor in vitro and in vivo. To this end chemical relaxation studies employing light-directed generation of ATP from caged ATP
Light-triggered strand exchange reaction using the change in the hydrogen bonding pattern of a nucleobase analogue.
Morihiro K, et al.
Chemical Science, 5(2), 744-750 (2014)
Synthesis and Characterizations of Positive-Working Photosensitive Polyimides Having 4, 5-Dimethoxy-o-Nitrobenzyl Side Group.
Ryu S, et al.
Bull. Korean Chem. Soc., 29(9), 1689-1689 (2008)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.