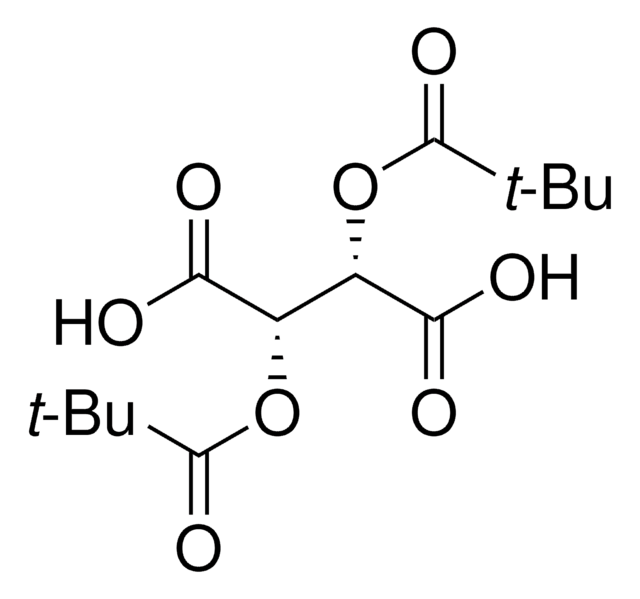
358924
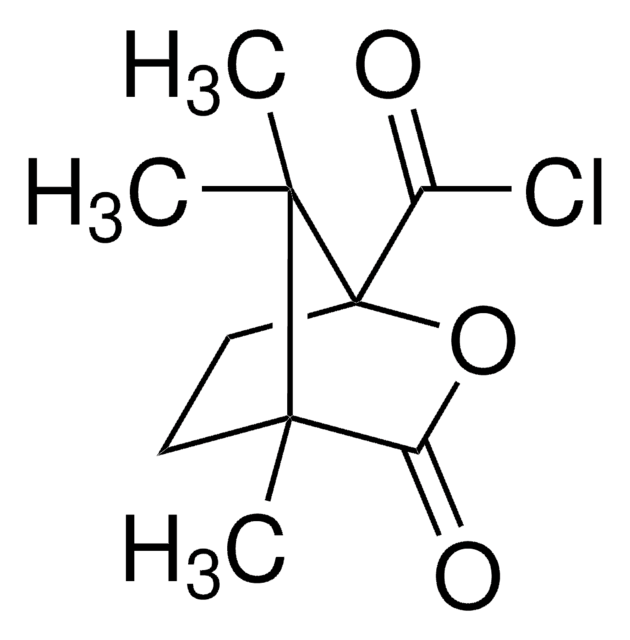
(+)-O,O′-Diacetyl-L-tartaric anhydride
97%
Sinonimo/i:
(+)-Diacetyl-L-tartaric anhydride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H8O7
Numero CAS:
Peso molecolare:
216.14
Beilstein:
87315
Numero CE:
Numero MDL:
Codice UNSPSC:
12352005
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Attività ottica
[α]20/D +59°, c = 6 in acetone
Punto di fusione
130-135 °C (lit.)
Gruppo funzionale
anhydride
ester
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(=O)O[C@@H]1[C@@H](OC(C)=O)C(=O)OC1=O
InChI
1S/C8H8O7/c1-3(9)13-5-6(14-4(2)10)8(12)15-7(5)11/h5-6H,1-2H3/t5-,6-/m1/s1
XAKITKDHDMPGPW-PHDIDXHHSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
(+)-O,O′-Diacetyl-L-tartaric anhydride is an HPLC derivatization reagent for UV/Vis detection. It is mainly employed as a reagent for the chiral derivatization of amino alcohols. It also reacts with alkanoamines in aprotic medium containing trichloroacetic acid and produces tartaric acid monoesters.
Applicazioni
(+)-O,O′-Diacetyl-L-tartaric anhydride may be used as a chiral derivatizating agent in the following:
- determination of enantiomeric vigabatrin in mouse serum samples using ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UHPLC-Q-TOF-M)
- determination of trantinterol in rat plasma by ultra performance liquid chromatography–electrospray ionization mass spectrometry (UPLC–MS/MS)
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Determination of enantiomeric vigabatrin by derivatization with diacetyl-l-tartaric anhydride followed by ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry
Zhao J, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 1040, 199-207 (2017)
D R Brocks et al.
Journal of pharmaceutical and biomedical analysis, 13(7), 911-918 (1995-06-01)
A stereospecific liquid chromatographic (LC) assay was developed for the quantification of the antimalarial drug, halofantrine, in human plasma. Following protein precipitation with acetonitrile, the enantiomers of halofantrine were extracted from human plasma using ammonium hydroxide and tert-butyl methyl ether-hexane.
D R Brocks et al.
Journal of chromatography, 581(1), 83-92 (1992-10-02)
(+/-)-Hydroxychloroquine (HCQ) is an antimalarial and anti-arthritic drug which is administered as the racemate. An accurate, precise and sensitive high-performance liquid chromatographic assay was developed for the determination of HCQ enantiomers in samples from human plasma, serum, whole blood, and
William M Oldham et al.
Bio-protocol, 6(16) (2017-06-03)
Two enantiomers of 2-hydroxyglutarate (2HG), L (L2HG) and D (D2HG), are metabolites of unknown function in mammalian cells that were initially associated with separate and rare inborn errors of metabolism resulting in increased urinary excretion of 2HG linked to neurological
W Lindner et al.
Journal of chromatography, 487(2), 375-383 (1989-02-24)
A sensitive high-performance liquid chromatographic method was developed for the stereoselective assay of (R)- and (S)-propranolol in human plasma. The method involves diethyl ether extraction of the drugs and a racemic internal standard, N-tert.-butylpropranolol, followed by derivatization of the compounds
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.