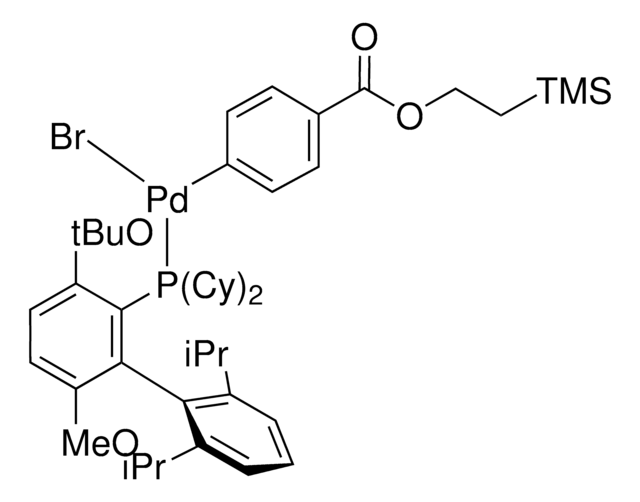
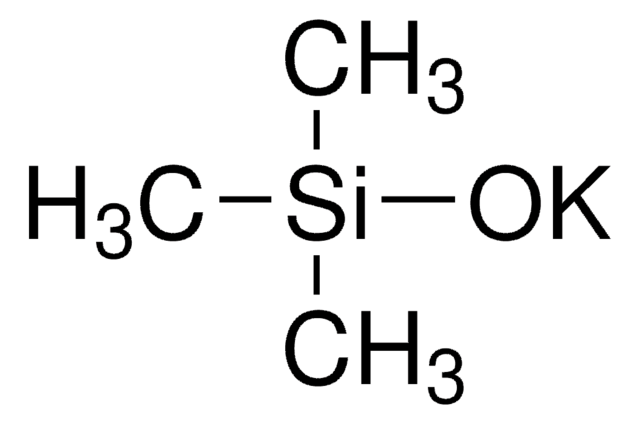
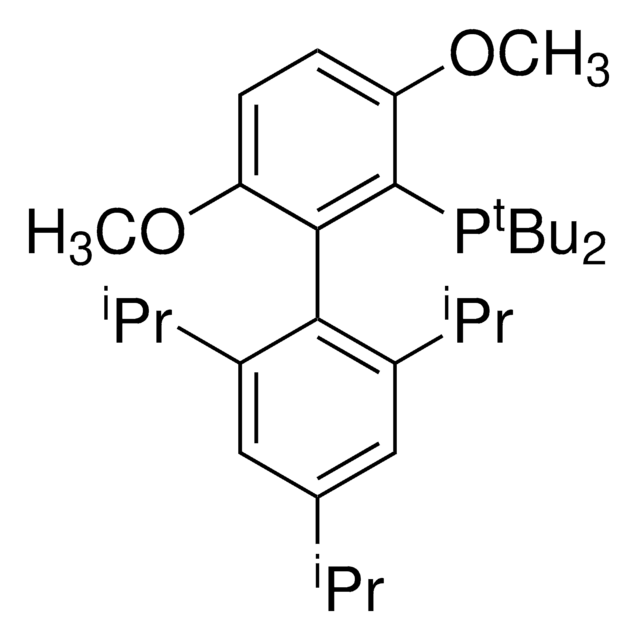
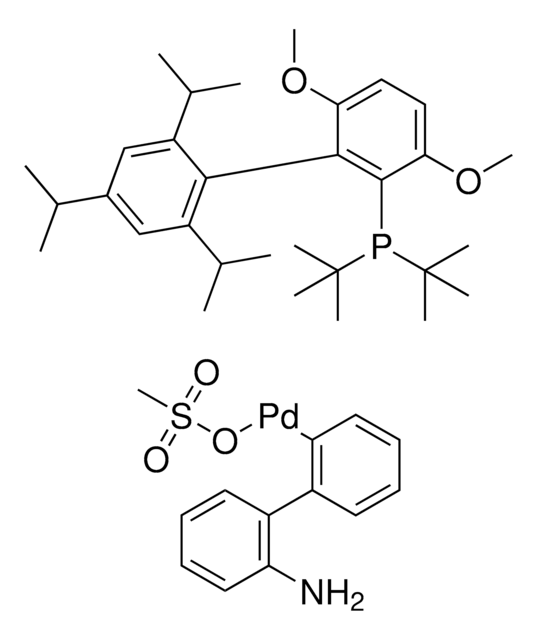
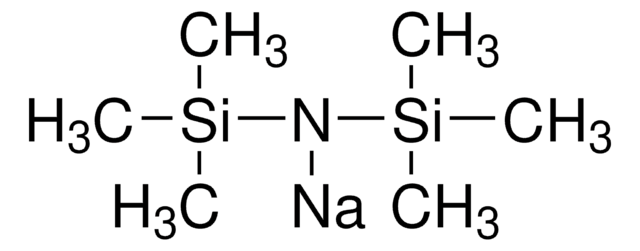329576
Sodium trimethylsilanolate
95%
Sinonimo/i:
Trimethylsilanol sodium salt
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
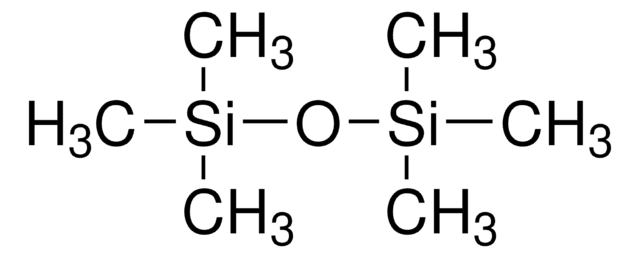
Formula condensata:
(CH3)3SiONa
Numero CAS:
Peso molecolare:
112.18
Beilstein:
3912148
Numero CE:
Numero MDL:
Codice UNSPSC:
12352000
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Punto di fusione
230 °C (dec.) (lit.)
Stringa SMILE
[Na+].C[Si](C)(C)[O-]
InChI
1S/C3H9OSi.Na/c1-5(2,3)4;/h1-3H3;/q-1;+1
HSNUIYJWTSJUMS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Sodium trimethylsilanolate is a versatile and very powerful reagent for the conversion of esters to carboxylic acids and the hydrolysis of nitriles to primary amides. It can be used as a starting material for the synthesis of metal silanolates via the salt metathesis and as a catalyst for the silylation of silanols with hydrosilanes.
Applicazioni
Sodium trimethylsilanolate is used as a catalyst in the:
- Synthesis of the rhodium silonate complex.
- Silylation of methylphenylsilane with tert-butyldimethylsilanol to synthesize trisiloxanes.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Scope and limitations of sodium and potassium trimethylsilanolate as reagents for conversion of esters to carboxylic acids
Lovric M, et al.
Croatica Chemica Acta. Arhiv Za Kemiju, 80, 109-115 (2007)
D M Hui et al.
Clinica chimica acta; international journal of clinical chemistry, 302(1-2), 171-188 (2000-11-14)
We developed a new assay method for fluoride anion (F(-)) a specific metabolite of sarin. Trimethyifluorosilane (TMFS) was derivatized from F(-) with trimethylsilanol, and TMFS was detected with a GC-flame ionization detector (FID) and capillary column system. The linear range
A Isquith et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(3), 263-266 (1988-03-01)
Six organosilicon compounds which had been found to have clastogenic activity in an in vitro battery of genotoxicity assays were evaluated in rat bone marrow cytogenetic assays for assessing clastogenicity in an in vivo system. None of the six compounds
Igor S Ignatyev et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(5), 1169-1178 (2004-04-16)
The assignment of the SiOH group vibrations of trimethylsilanol, which is still controversial, is proposed. This assignment is based on theoretical B3LYP force field scaled using the constants of the (CH3)3Si group optimized to fit experimental vibrational frequencies of (CH3)3SiF
Fabricio A Hansel et al.
Rapid communications in mass spectrometry : RCM, 25(13), 1893-1898 (2011-06-04)
A methodology is presented for the determination of dihydroxy fatty acids preserved in the 'bound' phase of organic residues preserved in archaeological potsherds. The method comprises saponification, esterification, silica gel column chromatographic fractionation, and analysis by gas chromatography/mass spectrometry. The
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.