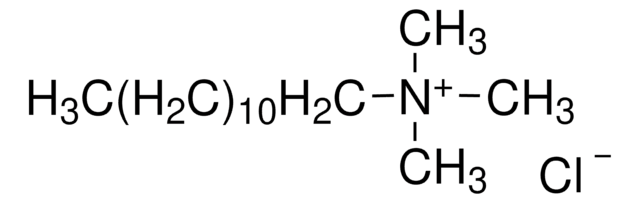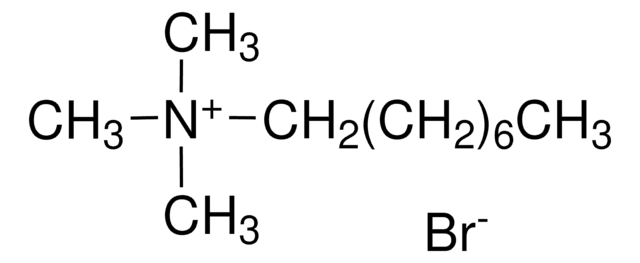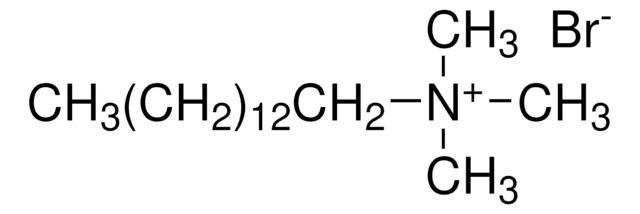30725
Decyltrimethylammonium bromide
≥98.0% (NT)
Sinonimo/i:
N,N,N-Trimethyl-1-decanaminium bromide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
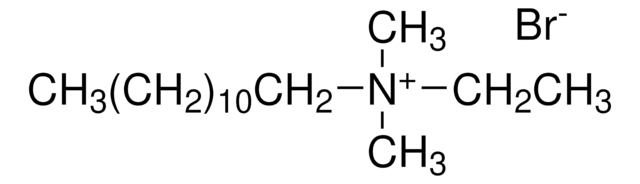
Formula condensata:
CH3(CH2)9N(CH3)3(Br)
Numero CAS:
Peso molecolare:
280.29
Beilstein:
3915222
Numero CE:
Numero MDL:
Codice UNSPSC:
12352116
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥98.0% (NT)
Stringa SMILE
[Br-].CCCCCCCCCC[N+](C)(C)C
InChI
1S/C13H30N.BrH/c1-5-6-7-8-9-10-11-12-13-14(2,3)4;/h5-13H2,1-4H3;1H/q+1;/p-1
PLMFYJJFUUUCRZ-UHFFFAOYSA-M
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Miguel Jorge
Langmuir : the ACS journal of surfaces and colloids, 24(11), 5714-5725 (2008-05-06)
In this paper, a molecular dynamics simulation of surfactant self-assembly using realistic atomistic models is presented. The simulations are long enough to enable the observation of several processes leading to equilibrium, such as monomer addition and detachment, micelle dissolution, and
Zsombor Feldötö et al.
Langmuir : the ACS journal of surfaces and colloids, 24(7), 3348-3357 (2008-02-13)
The interaction between mucin and ions has been investigated by employing the quartz crystal microbalance technique with measurement of energy dissipation. The study was partially aimed at understanding the adsorption of mucin on surfaces with different chemistry, and for this
Nina M Kovalchuk et al.
Langmuir : the ACS journal of surfaces and colloids, 35(28), 9184-9193 (2019-07-04)
The coalescence of two different drops, one surfactant-laden and the other surfactant-free, was studied under the condition of confined flow in a microchannel. The coalescence was accompanied by penetration of the surfactant-free drop into the surfactant-laden drop because of the
Qian Zhao et al.
Environmental science & technology, 46(7), 3999-4007 (2012-03-01)
Organoclays synthesized from single chain quaternary ammonium cations (QAC) ((CH(3))(3)NR(+)) exhibit different mechanisms for the sorption of nonpolar organic compounds as the length of the carbon chain is increased. The interaction between a nonpolar sorbate and an organoclay intercalated with
Jonas Carlstedt et al.
Langmuir : the ACS journal of surfaces and colloids, 28(5), 2387-2394 (2012-01-06)
Full equilibrium phase diagrams are presented for two ternary systems composed of the cationic surfactant dodecyltrimethylammonium bromide (DTAB), water (D(2)O), and a cyclodextrin, either β-cyclodextrin (β-CD) or (2-hydroypropyl)-β-cyclodextrin (2HPβCD). (2)H NMR, SAXS, WAXS, and visual examination were used to determine
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.