291110
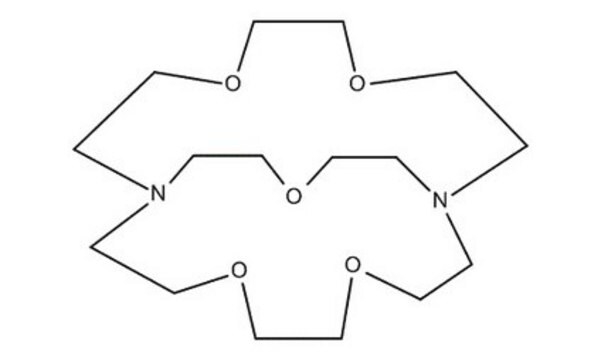
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane
98%
Sinonimo/i:
Cryptand 222, Kryptofix® 222
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C18H36N2O6
Numero CAS:
Peso molecolare:
376.49
Beilstein:
620282
Numero CE:
Numero MDL:
Codice UNSPSC:
12352005
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
solid
Punto di fusione
68-71 °C (lit.)
Stringa SMILE
C1COCCN2CCOCCOCCN(CCO1)CCOCCOCC2
InChI
1S/C18H36N2O6/c1-7-21-13-14-24-10-4-20-5-11-25-17-15-22-8-2-19(1)3-9-23-16-18-26-12-6-20/h1-18H2
AUFVJZSDSXXFOI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane(cryptand 222, Kryptofix 222) is an organic compound with formula C18H36N2O6. This compound has a cage-like three-dimensional structure that donates N2O6. The ligand has shown a wide range of uses in magnetic resonance imaging, organic synthesis, crystallography, electrochemistry, and chromatography. In solution, it is a well-known sequestering agent for metal ions.
Applicazioni
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane is used as:
- A electrolyte additive in isotachophoresis[separation method that is particularly suited to the analysis of small ions]for the analysis of alkali metal cations.
- A complexing agent in the preparation of K+-imprinted nanoparticles using methacrylic acid as the functional monomer, ethylene glycol dimethacrylate as the crosslinker and 2,2′-azobisisobutyronitrile as the radical initiator.
- A structure directing agent (SDA) in the preparation of LTA-type AlPO4 crystals.
Confezionamento
Bottomless glass bottle. Contents are inside inserted fused cone.
Cryptand used with potassium mirror to reduce a hindered distannene to a crystalline radical anion.
Note legali
Kryptofix is a registered trademark of Merck KGaA, Darmstadt, Germany
Applicazioni
N° Catalogo
Descrizione
Determinazione del prezzo
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Metal Ion Compiexing by Cryptand 222 in Solutions. A Thermodynamic Approach
Marcus Y
Reviews in Analytical Chemistry, 23, 269-302 (2004)
Preparation of large and well-shaped LTA-type AlPO4 crystals by using crown ether Kryptofix 222 as structure directing agent
Huang A, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 129, 90-99 (2010)
Vladimir Ya Lee et al.
Journal of the American Chemical Society, 128(35), 11643-11651 (2006-08-31)
((t)Bu(2)MeSi)(2)Sn=Sn(SiMe(t)Bu(2))(2) 1, prepared by the reaction of (t)Bu(2)MeSiNa with SnCl(2)-diox in THF and isolated as dark-green crystals, represents the first example of acyclic distannene with a Sn=Sn double bond that is stable both in the crystalline form and in solution.
T Colomina et al.
Veterinary and human toxicology, 33(2), 121-124 (1991-04-01)
The effects of repeated ip administration of diethylenetriaminepentaacetic acid (DTPA), Kryptofix 222, 1,4,7,10,13,16-hexaoxacyclooctadecane (18-crown-6), ethylenglycol-bis-(beta-amino-ethylether)-N,N'-tetraacetic acid (EGTA), and Kryptofix 5 on the distribution and excretion of sc-injected strontium were investigated in male Swiss mice. Groups of 20 animals received 95
T Chaly et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 16(4), 385-387 (1989-01-01)
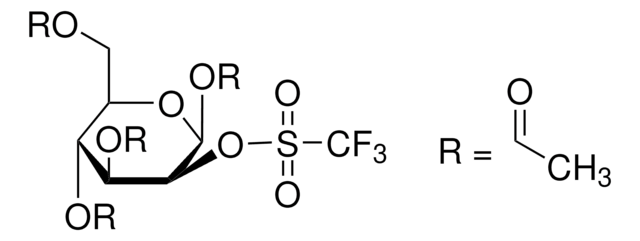
A simple procedure for the detection of 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo (8.8.8) hexacosane (Kryptofix 2.2.2) in the final solution of [18F]FDG prepared by the aminopolyether supported nucleophilic substitution of 1,3,4,6-tetra-O-acetyl 2-O-trifluoromethanesulfonyl-beta-D-mannopyranose (Hamacher et al., 1986) has been developed. Presence of Kryptofix 2.2.2 in
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 291110-10G | |
| 291110-10X22MG | |
| 291110-250MG | 4061826552766 |
| 291110-5G | |
| 291110-1G | 4061835554928 |
| 291110-10X20MG | |
| 291110-15MG | |
| 291110-16MG | |
| 291110-20MG | |
| 291110-22MG | |
| 291110-250G |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.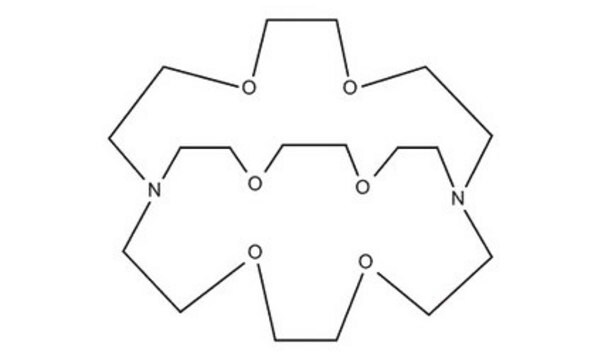

![4,7,13,16,21-Pentaoxa-1,10-diazabicyclo[8.8.5]tricosane 98%](/deepweb/assets/sigmaaldrich/product/structures/444/464/eeb08f63-862e-447e-8e41-342d713f439c/640/eeb08f63-862e-447e-8e41-342d713f439c.png)