286958
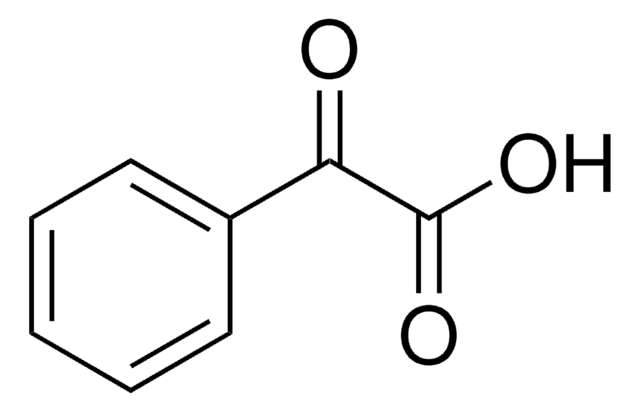
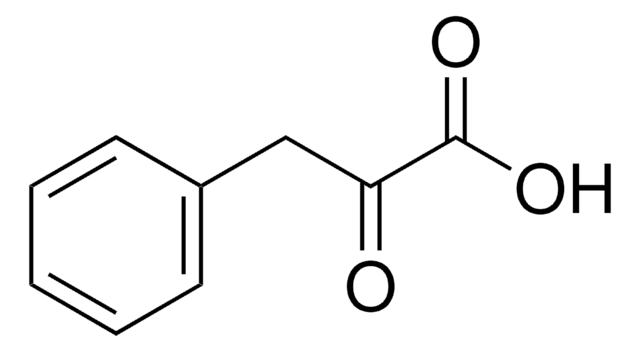
Phenylpyruvic acid
98%
Sinonimo/i:
2-Oxo-3-phenylpropanoic acid, 2-Oxo-3-phenylpropionic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
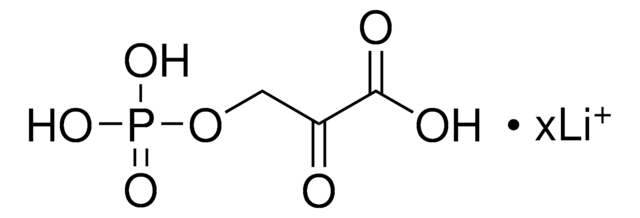
Formula condensata:
C6H5CH2COCOOH
Numero CAS:
Peso molecolare:
164.16
Beilstein:
2207312
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
150-154 °C (lit.)
Gruppo funzionale
carboxylic acid
ketone
phenyl
Temperatura di conservazione
−20°C
Stringa SMILE
OC(=O)C(=O)Cc1ccccc1
InChI
1S/C9H8O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,11,12)
BTNMPGBKDVTSJY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Phenylpyruvic acid reduces glucose-6-phosphate dehydrogenase activity without pre-incubation.
Applicazioni
Phenylpyruvic acid was used in the synthesis of 3-phenyllactic acid (PLA) by lactate dehydrogenase.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Shuhuai Yu et al.
Biotechnology letters, 36(3), 627-631 (2013-11-20)
3-Phenyllactic acid (PLA) is an antimicrobial compound with broad and effective antimicrobial activity against both bacteria and fungi. Enzymatic production of PLA can be carried out from phenylpyruvic acid by lactate dehydrogenase (LDH); however, the enzymatic reaction is accompanied by
Taiki Fujii et al.
Biochimica et biophysica acta, 1814(12), 1669-1676 (2011-06-16)
We discovered the phenyllactate (PLA)-producing fungal strain Wickerhamia fluorescens TK1 and purified phenylpyruvate reductase (PPR) from fungal cell-free extracts. The PPR used both NADPH and NADH as cofactors with more preference for the former. The enzyme reaction as well as
Andrea Pereira Rosa et al.
Cellular and molecular neurobiology, 32(7), 1113-1118 (2012-04-06)
Phenylketonuria is a recessive autosomal disorder that is caused by a deficiency in the activity of phenylalanine-4-hydroxylase, which converts phenylalanine to tyrosine, leading to the accumulation of phenylalanine and its metabolites phenyllactic acid, phenylacetic acid, and phenylpyruvic acid in the
A Hargreaves et al.
Journal of photochemistry and photobiology. B, Biology, 89(2-3), 110-116 (2007-11-06)
Ultraviolet A (UVA) light (315-400 nm) is ubiquitously found in our environment and constitutes about 95% of the total solar UV; all UVC and most UVB being absorbed by the stratospheric ozone layer. Compared with UVB and C, UVA does
Faqing Tang et al.
Clinical biochemistry, 44(8-9), 711-718 (2011-03-16)
To search for markers of nasopharyngeal carcinoma (NPC) for diagnosis. Using gas chromatography and mass spectrometry, we evaluated 51 serum metabolites in 49 NPC, 37 throat cancer patients and 40 healthy controls. High metabolites were selected and confirmed in NPC
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.