260517
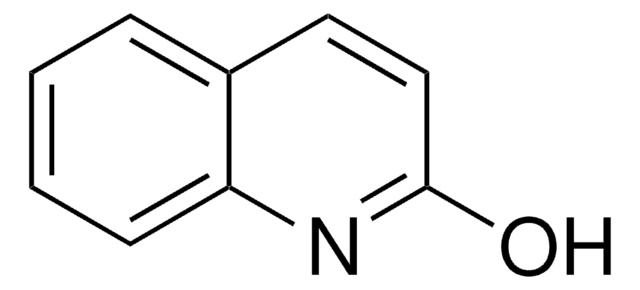
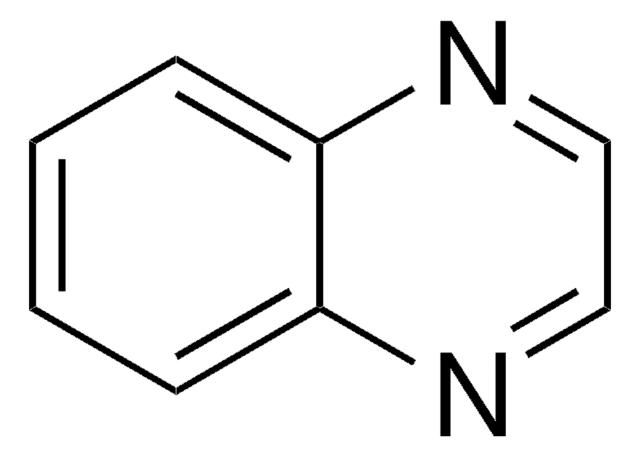
2-Quinoxalinol
99% (HPLC)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
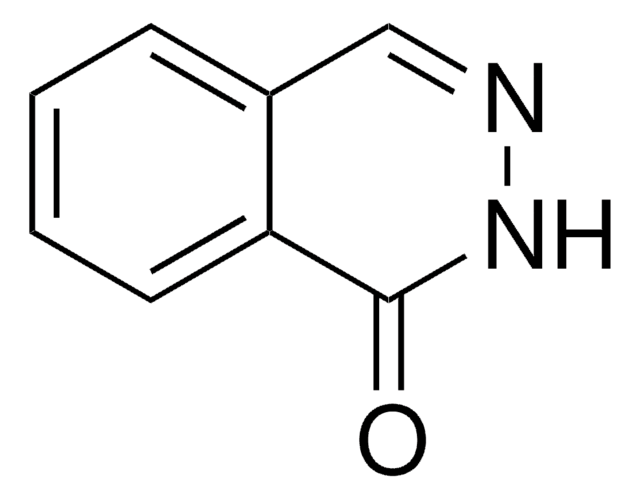
Formula empirica (notazione di Hill):
C8H6N2O
Numero CAS:
Peso molecolare:
146.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99% (HPLC)
Punto di fusione
271-272 °C (lit.)
Stringa SMILE
Oc1cnc2ccccc2n1
InChI
1S/C8H6N2O/c11-8-5-9-6-3-1-2-4-7(6)10-8/h1-5H,(H,10,11)
FFRYUAVNPBUEIC-UHFFFAOYSA-N
Descrizione generale
Epitaxial crystallization of syndiotactic polypropylene on 2-quinoxalinol yields isochiral form II of syndiotactic polypropylene. 2-Quinoxalinol participates in direct dehydrative cross-coupling of 2-quinoxalinone with p-tolylacetylene via Pd/Cu-catalyzed phosphonium coupling.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Pramila Menon et al.
Chemosphere, 53(8), 1023-1031 (2003-09-25)
The dissipation of 14C carbaryl in undisturbed soil cores, and of quinalphos (25EC and 20AF) after seed and soil treatments, was investigated under field use conditions, in a semi-arid groundnut field. Residues were analyzed by TLC and HPLC and additionally
R Q He et al.
Biochemistry and molecular biology international, 37(3), 447-457 (1995-10-01)
A procedure for transaminating proteins and removing the transaminated N-terminal residue has been used for studying structure-function relationship of protein (Dixon and Fields 1972, Meth. Enzymol. 25, 409-419). We show that it is convenient for measuring the relative molecular masses
Isochiral form II of syndiotactic polypropylene produced by epitaxial crystallization.
Zhang J, et al.
Macromolecules, 34(18), 6261-6267 (2001)
Fu-An Kang et al.
Chemical communications (Cambridge, England), 46(8), 1347-1349 (2010-05-08)
The first chemoselective direct dehydrative cross-coupling of tautomerizable heterocycles with alkynes has been achieved via C-H/C-OH bond activations with direct C(sp(2))-C(sp) bond formation, which is in line with ideal synthesis using readily available materials.
Andreas Behrends et al.
Redox report : communications in free radical research, 9(5), 279-288 (2004-12-21)
Toxicity of the pesticide quinalphos may comprise secondary, delayed effects by its main metabolite 2-hydroxyquinoxaline (HQO). We demonstrate that HQO can destroy photocatalytically vitamins C and E, catecholamines, serotonin, melatonin, the melatonin metabolite AMK (N(1)-acetyl-5-methoxykynuramine), and unsubstituted and substituted anthranilic
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.