243086
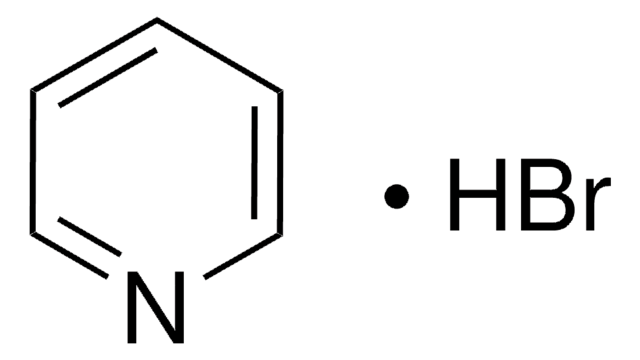
Pyridine hydrochloride
98%
Sinonimo/i:
Pyridinium chloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H5N · HCl
Numero CAS:
Peso molecolare:
115.56
Beilstein:
3615340
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
39151701
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
P. ebollizione
222-224 °C (lit.)
Punto di fusione
145-147 °C (lit.)
Solubilità
ethanol: soluble 50 mg/mL, clear, colorless to light yellow
Stringa SMILE
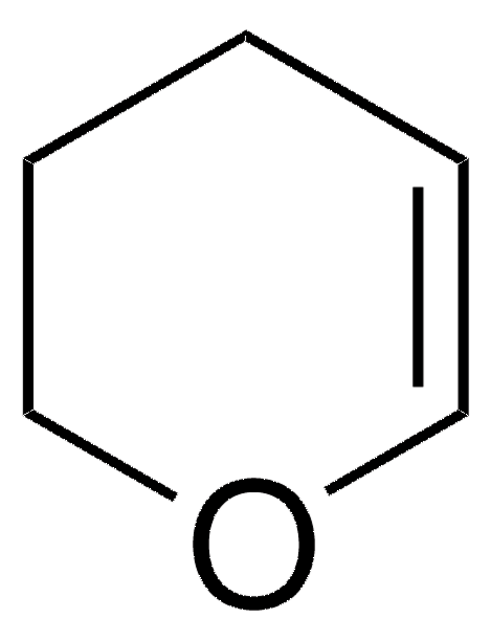
Cl[H].c1ccncc1
InChI
1S/C5H5N.ClH/c1-2-4-6-5-3-1;/h1-5H;1H
AOJFQRQNPXYVLM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Pyridine hydrochloride is an acidic type demethylating agent used as a catalyst in deprotection of aromatic methyl ethers.
Applicazioni
Pyridine hydrochloride (pyridine hydrochloride) was used in the demethylation of 4,5-dimethyl-7-methoxy-1-tetralone.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Demethylation of methyl aryl ethers using pyridine hydrochloride in solvent-free conditions under microwave irradiation.
Kulkarni PP, et al.
J. Chem. Res. Synop., 6, 394-395 (1999)
Tatyana N Sevastyanova et al.
Molecular pharmacology, 86(5), 492-504 (2014-08-13)
Metabotropic glutamate receptors (mGluRs) function as dimers. Recent work suggests that mGluR1 and mGluR5 may physically interact, but the nature and functional consequences of this relationship have not been addressed. In this study, the functional and pharmacological consequences of this
Nathalie Ségaud et al.
Inorganic chemistry, 52(2), 691-700 (2013-01-11)
We report the synthesis, characterization, and solution chemistry of a series of new Fe(II) complexes based on the tetradentate ligand N-methyl-N,N'-bis(2-pyridyl-methyl)-1,2-diaminoethane or the pentadentate ones N,N',N'-tris(2-pyridyl-methyl)-1,2-diaminoethane and N,N',N'-tris(2-pyridyl-methyl)-1,3-diaminopropane, modified by propynyl or methoxyphenyltriazolyl groups on the amino functions. Six of
Jipan Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4271-4277 (2013-02-13)
Efficient copper-catalyzed aerobic oxidative C-H and C-C functionalization of 1-[2-(arylamino)aryl]ethanones leading to acridones has been developed. The procedure involves cleavage of aromatic C-H and acetyl C-C bonds with intramolecular formation of a diarylketone bond. The protocol uses inexpensive Cu(O2CCF3)2 as
Ye Wei et al.
Journal of the American Chemical Society, 135(10), 3756-3759 (2013-02-27)
We describe here a [3+3]-type condensation reaction of O-acetyl ketoximes and α,β-unsaturated aldehydes that is synergistically catalyzed by a copper(I) salt and a secondary ammonium salt (or amine). This redox-neutral reaction allows modular synthesis of a variety of substituted pyridines
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.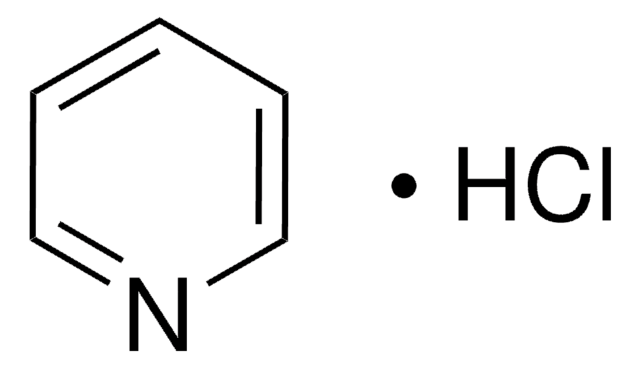



![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)
