206555
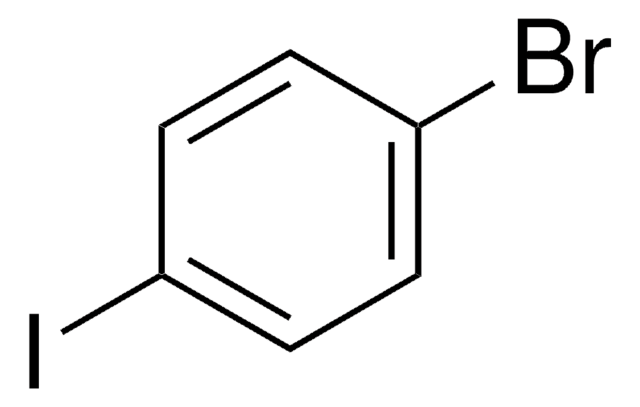
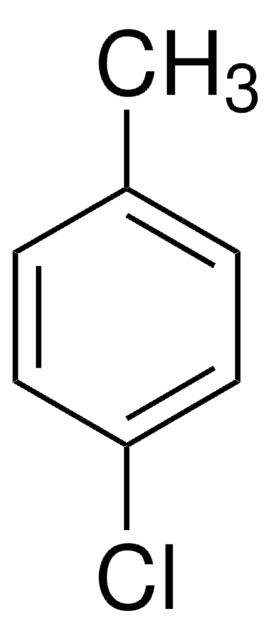
4-Iodotoluene
99%
Sinonimo/i:
1-Iodo-4-methylbenzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3C6H4I
Numero CAS:
Peso molecolare:
218.03
Beilstein:
1903637
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
solid
P. ebollizione
211.5 °C (lit.)
Punto di fusione
33-35 °C (lit.)
Gruppo funzionale
iodo
Stringa SMILE
Cc1ccc(I)cc1
InChI
1S/C7H7I/c1-6-2-4-7(8)5-3-6/h2-5H,1H3
UDHAWRUAECEBHC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
4-Iodotoluene undergoes Suzuki-Miyaura coupling reaction with phenylboronic acid catalyzed by (Ni,Mg)3Si2O5(OH)4 solid-solution nanotubes loaded with palladium. Cobalt-catalyzed coupling of 4-iodotoluene with thiophenols and alkanethiols has been investigated. Palladium/copper-catalyzed Sonogashira cross-coupling reaction of 4-iodotoluene with phenylacetylene has been studied.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
194.0 °F - closed cup
Punto d’infiammabilità (°C)
90 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Wancheng Zhu et al.
Inorganic chemistry, 51(11), 6020-6031 (2012-05-16)
(Ni(1-x),Mg(x))(3)Si(2)O(5)(OH)(4) solid-solution nanotubes (NTs) with tunable compositions were hydrothermally synthesized by altering the molar ratio of Mg(2+) to Ni(2+). The as-synthesized NTs were loaded with sub-0.06 wt % palladium (Pd; ∼0.045 wt %) for Suzuki-Miyaura (SM) coupling reactions between iodobenzene
Takashi Mino et al.
The Journal of organic chemistry, 71(25), 9499-9502 (2006-12-02)
Palladium/copper-catalyzed Sonogashira cross-coupling reaction of aryl halides with a variety of terminal alkynes under amine-free conditions in dimethylformamide (DMF) at 80 degrees C gave internal arylated alkynes using PdCl2(MeCN)2 with phosphine-free hydrazone 2a as a ligand and CuI as the
Ying-Chieh Wong et al.
Organic letters, 8(24), 5613-5616 (2006-11-17)
A new cobalt-catalyzed coupling of aryl halides with thiophenols and alkanethiols is reported. A variety of aryl sulfides can be prepared in excellent yields under mild reaction conditions using 1-2 mol % of CoI2(dppe) and Zn. This new cobalt-catalyzed coupling
V Kolaříková et al.
Dalton transactions (Cambridge, England : 2003), 44(45), 19663-19673 (2015-09-17)
Using three different approaches, racemic 1-(perfluoroalkyl)ethylamines were synthesized from perfluoroalkyl iodides or perfluoroalkanoic acids, and further transformed to the corresponding N,N'-disubstituted ethane-1,2-diimines and ethane-1,2-diamines as mixtures of diastereoisomers. Their cyclization afforded imidazolium or dihydroimidazolium salts, which led to silver or
Zengyan Wei et al.
Nature communications, 5, 3870-3870 (2014-05-16)
The shape-controlled synthesis of nanoparticles was established in single-phase solutions by controlling growth directions of crystalline facets on seed nanocrystals kinetically; however, it was difficult to rationally predict and design nanoparticle shapes. Here we introduce a methodology to fabricate nanoparticles
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)