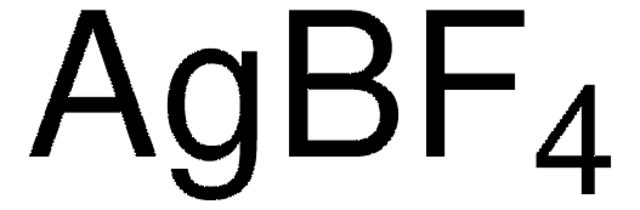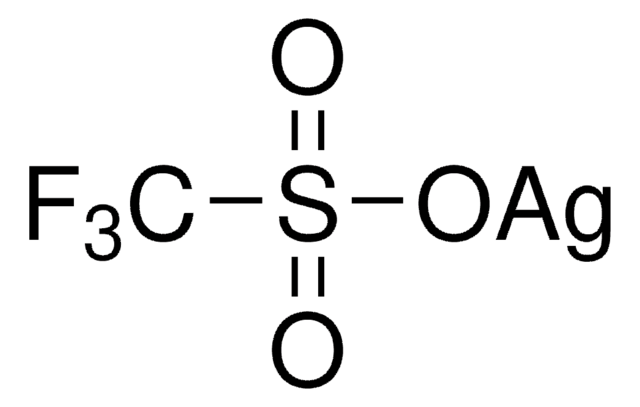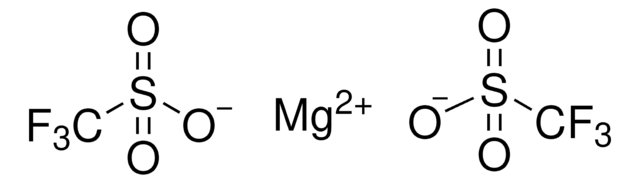176435
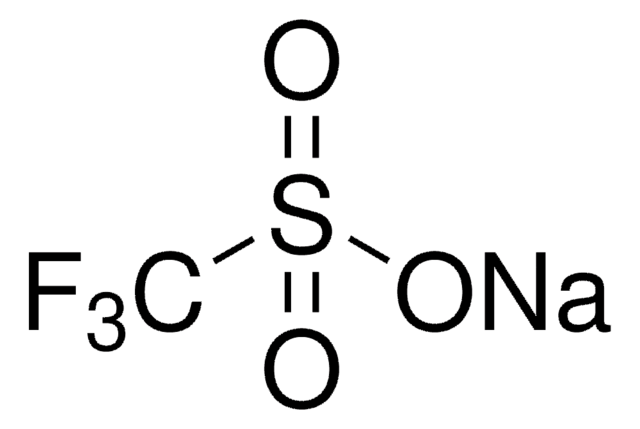
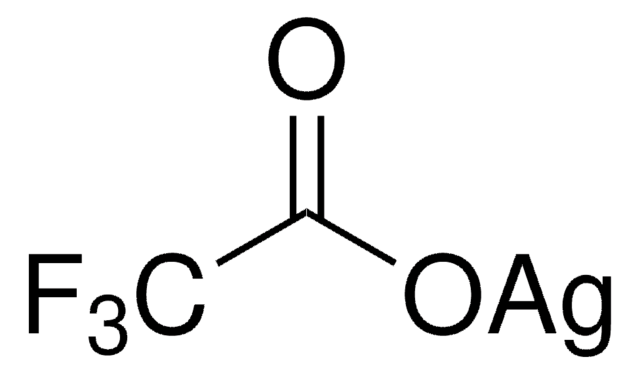
Silver trifluoromethanesulfonate
≥99%
Sinonimo/i:
Silver triflate, Trifluoromethanesulfonic acid silver salt
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Stato
powder
Impiego in reazioni chimiche
core: silver
reagent type: catalyst
Punto di fusione
286 °C (lit.)
Stringa SMILE
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
QRUBYZBWAOOHSV-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
It can also be used:
- To obtain olefins from secondary phosphates and thiophosphates.
- As a reagent in the etherification of alcohols with primary alkyl halides under mild conditions.
- To generate cationic rhodium catalysts from chlororhodium complexes for the hydrophosphination of acetylenes.
- As a catalyst for the preparation of silyl ethers by hydrosilylation of aldehydes.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Dam. 1 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.