164771
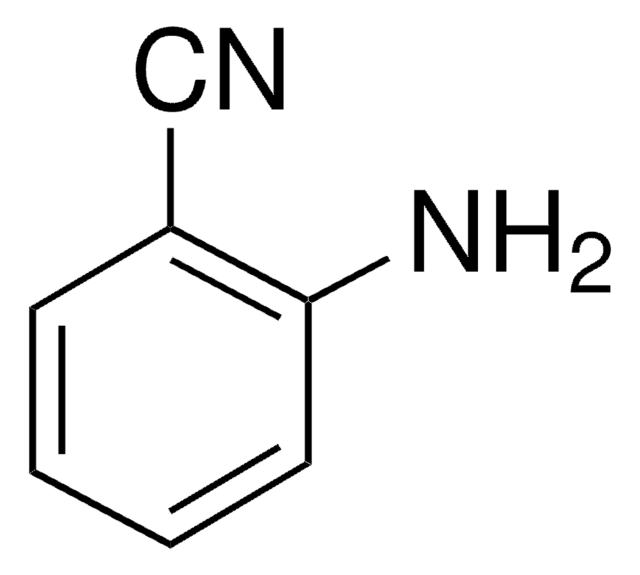
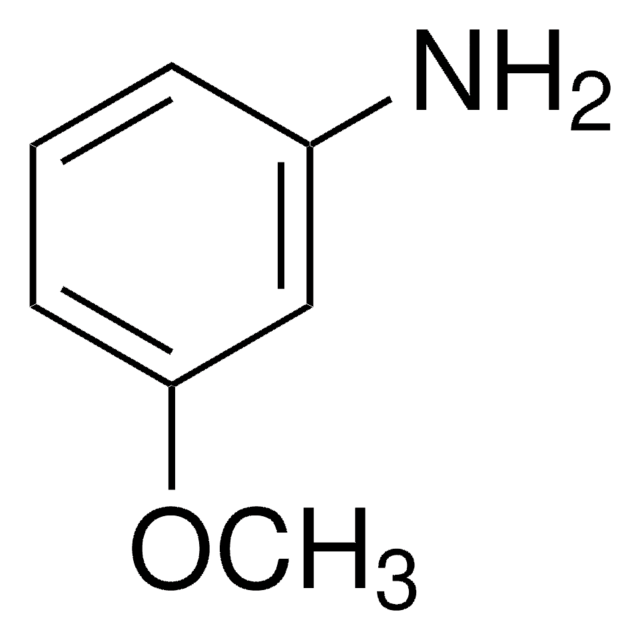
3-Aminobenzonitrile
99%
Sinonimo/i:
3-Cyanoaniline
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
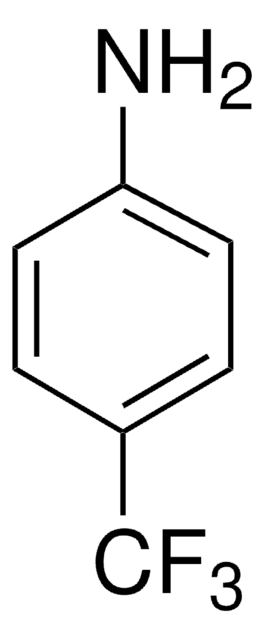
Formula condensata:
H2NC6H4CN
Numero CAS:
Peso molecolare:
118.14
Beilstein:
636498
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
solid
Punto di fusione
48-53 °C (lit.)
Gruppo funzionale
nitrile
Stringa SMILE
Nc1cccc(c1)C#N
InChI
1S/C7H6N2/c8-5-6-2-1-3-7(9)4-6/h1-4H,9H2
NJXPYZHXZZCTNI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
3-Aminobenzonitrile on condensation reaction with 4-isothiocyanato-4-methyl pentane-2-one gives condensed monocyclic pyrimidine derivatives.
Applicazioni
3-Aminobenzonitrile was used in the synthesis of series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles. It was also used in the preparation of highly substituted γ-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Sens. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
233.6 °F - closed cup
Punto d’infiammabilità (°C)
112 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Cheng Hua Jin et al.
European journal of medicinal chemistry, 46(9), 3917-3925 (2011-06-24)
A series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles 14a-d, 15a-d, 17a, 17b, 18a-d, 19a, and 19b has been synthesized and evaluated for their ALK5 inhibitory activity in an enzyme assay and in a cell-based luciferase reporter assay. The 2-[3-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl]-N-phenylethanethioamide (18a) inhibited ALK5 phosphorylation with
Sham M Sondhi et al.
Bioorganic & medicinal chemistry, 13(22), 6158-6166 (2005-08-24)
3-Aminobenzonitrile and 2-amino-4-phenyl thiazole on condensation with 4-isothiocyanato-4-methyl pentane-2-one gave condensed monocyclic pyrimidine derivatives 1 and 2, 3, respectively. Condensation of 3-aminopropyl imidazole with 3-isothiocyantobutanal gave condensed monocyclic pyrimidine derivative 4. Bicyclic pyrimidine derivatives 5a and 5b have been synthesized
Jeffrey C Pelletier et al.
Bioorganic & medicinal chemistry, 13(21), 5986-5995 (2005-08-16)
An unusual combination of Weinreb amidation and Mitsunobu lactam formation was used to prepare highly substituted gamma-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist. The analogue synthesis was stereoselective and the final products were chemically stable. Biological properties
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.