159034
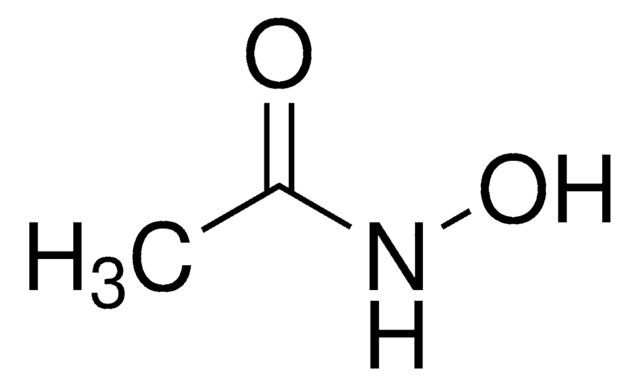
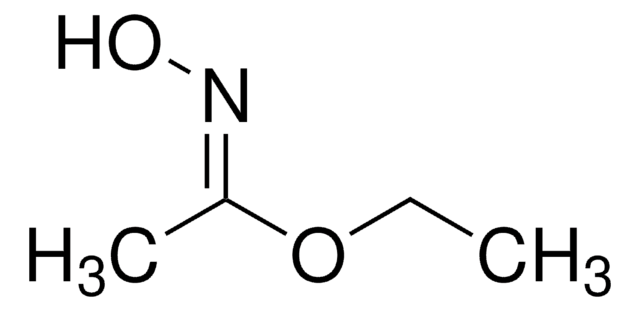
Acetohydroxamic acid
98%
Sinonimo/i:
AHA
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
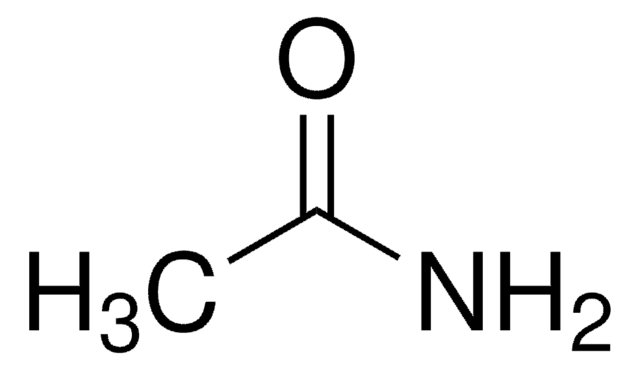
Formula condensata:
CH3CONHOH
Numero CAS:
Peso molecolare:
75.07
Beilstein:
1739019
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
88-90 °C (lit.)
Gruppo funzionale
amine
Stringa SMILE
CC(NO)=O
InChI
1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4)
RRUDCFGSUDOHDG-UHFFFAOYSA-N
Informazioni sul gene
human ... CA2(760) , MMP3(4314)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Acetohydroxamic acid is a potent inhibitor of bacterial urease activity and reduces urinary ammonia levels. 2-Acetohydroxamic acid loaded floating microspheres forms an efficient drug delivery system for the treatment of Helicobacter pylori.
Applicazioni
Acetohydroxamic acid was used:
- to study the mechanism of complexation of iron (III) with acetohydroxamic acid
- to study the inhibitory mechanism of lansoprazole and omeprazole on Helicobacter pyloni
- in urease inhibition studies
- for in situ generation of nitrosocarbonylmethane as a Diels-Alder dienophile
Used in urease inhibition studies and for in situ generation of nitrosocarbonylmethane as a Diels-Alder dienophile.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
K Nagata et al.
Antimicrobial agents and chemotherapy, 37(4), 769-774 (1993-04-01)
The gastric proton pump inhibitor lansoprazole, its active analog AG-2000, and omeprazole dose dependently inhibited urease activity extracted with distilled water from Helicobacter pylori cells; the 50% inhibitory concentrations were between 3.6 and 9.5 microM, which were more potent than
Zbl. Bakt., 275, 63-63 (1991)
D P Griffith et al.
The Journal of urology, 140(2), 318-324 (1988-08-01)
Acetohydroxamic acid is known to inhibit bacterial urease activity, thus, reducing urinary ammonia levels. A double-blind placebo-controlled clinical trial of acetohydroxamic acid was conducted at 12 Veterans Administration spinal cord injury units. A total of 210 male spinal cord injury
Journal of the Chemical Society. Perkin Transactions 1, 1001-1001 (1991)
Mechanism of iron (III) complex formation. Activation volumes for the complexation of the iron (III) ion with thiocyanate ion and acetohydroxamic acid.
Funahashi S, et al.
Inorganic Chemistry, 22(14), 2070-2073 (1983)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.