130222
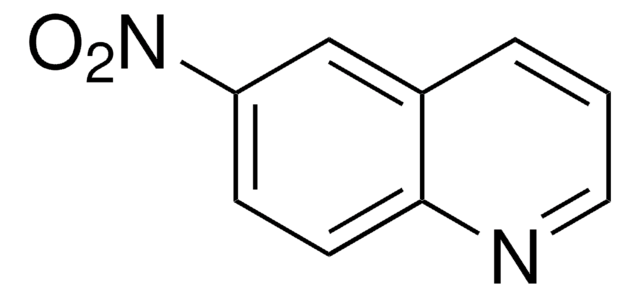
5-Nitroisoquinoline
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H6N2O2
Numero CAS:
Peso molecolare:
174.16
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Stato
powder
Punto di fusione
106-109 °C (lit.)
Stringa SMILE
[O-][N+](=O)c1cccc2cnccc12
InChI
1S/C9H6N2O2/c12-11(13)9-3-1-2-7-6-10-5-4-8(7)9/h1-6H
PYGMPFQCCWBTJQ-UHFFFAOYSA-N
Descrizione generale
5-Nitroisoquinoline reacted with vinylmagnesium bromide to form a number of pyrroloisoquinolines. 5-Nitroisoquinoline derivatives (potential antimalarial drugs) were evaluated for mutagenic (MUT) and chromosome-damaging (CHR) activities by the Salmonella test.
Applicazioni
5-Nitroisoquinoline was used to prepare a number of pyrroloisoquinolines.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Margarita Vlachou et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 17(3), 139-143 (2002-10-24)
A number of pyrroloisoquinolines have been prepared by reaction of 5-nitroisoquinoline with vinylmagnesium bromide followed by N-alkylation with the appropriate 2-chloro-N,N-dialkylethylamine. Their cytotoxicity was evaluated in a number of ovarian cell lines and compared to their analogous isomeric pyrroloquinolines. Two
T Orsière et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 41(2), 275-290 (2002-12-14)
The mutagenic (MUT) and chromosome-damaging (CHR) activities of 22 potential antimalarial drugs (5-nitroisoquinoline derivatives) were evaluated by the Salmonella test and the cytokinesis-blocked micronucleus assay (CBMN). The Salmonella mutagenicity test was performed with and without metabolic activation (S9 mix) in
Nelson R Vinueza et al.
Physical chemistry chemical physics : PCCP, 20(33), 21567-21572 (2018-08-11)
Two previously unreported isomeric biradicals with a 1,4-radical topology, the 1,5-didehydroisoquinolinium cation and the 4,8-didehydroisoquinolinium cation, and an additional, previously reported isomer, the 4,5-didehydroisoquinolinium cation, were studied to examine the importance of the exact location of the radical sites on
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.