126020
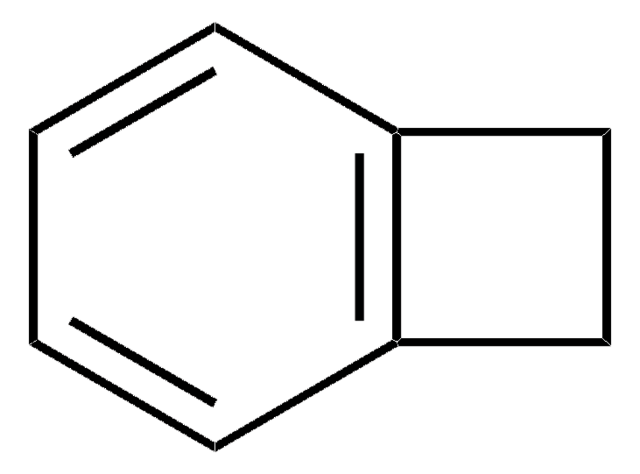
1,4-Dichlorophthalazine
95%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H4Cl2N2
Numero CAS:
Peso molecolare:
199.04
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Punto di fusione
160-162 °C (lit.)
Gruppo funzionale
chloro
Temperatura di conservazione
−20°C
Stringa SMILE
Clc1nnc(Cl)c2ccccc12
InChI
1S/C8H4Cl2N2/c9-7-5-3-1-2-4-6(5)8(10)12-11-7/h1-4H
ODCNAEMHGMYADO-UHFFFAOYSA-N
Categorie correlate
Applicazioni
1,4-Dichlorophthalazine was used as starting reagent in the synthesis of series of 4-aryl-1-(4-methylpiperazin-1-yl)phthalazines. It was used as coupling reagent in the synthesis of novel soluble polymer-bound ligand. It was often used as building block in medicinal chemistry synthesis.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
K De Wael et al.
Science & justice : journal of the Forensic Science Society, 55(6), 422-430 (2015-12-15)
Reactively-dyed black, navy blue and medium red cotton samples showing metamerism under fluorescent tube illumination were examined. Optical microscopy (bright field, polarization and fluorescence microscopy) was used, followed by microspectrometry in the visible range (MSP Vis), to differentiate the samples
Synthesis of 4-aryl-1-(4-methylpiperazin-1-yl) phthalazines by Suzuki-type cross-coupling reaction.
Guery S, et al.
Synthesis, 2001(05), 699-701 (2001)
Matthew A J Duncton et al.
Bioorganic & medicinal chemistry letters, 16(6), 1579-1581 (2006-01-03)
A novel class of 1-(isoquinolin-5-yl)-4-arylamino-phthalazines is described as inhibitors of vascular endothelial growth factor receptor II (VEGFR-2). Many compounds display VEGFR-2 inhibitory activity with an IC(50) as low as 0.017 microM in an HTRF enzymatic assay. The compounds also inhibit
A simple and effective soluble polymer-bound ligand for the asymmetric dihydroxylation of olefins: DHQD-PHAL-OPEG-OMe.
Kuang YQ, et al.
Tetrahedron Letters, 42(34), 5925-5927 (2001)
William H Bunnelle et al.
Journal of medicinal chemistry, 50(15), 3627-3644 (2007-06-26)
A series of exceptionally potent agonists at neuronal nicotinic acetylcholine receptors (nAChRs) has been investigated. Several N-(3-pyridinyl) derivatives of bridged bicyclic diamines exhibit double-digit-picomolar binding affinities for the alpha 4 beta 2 subtype, placing them with epibatidine among the most
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.