122882
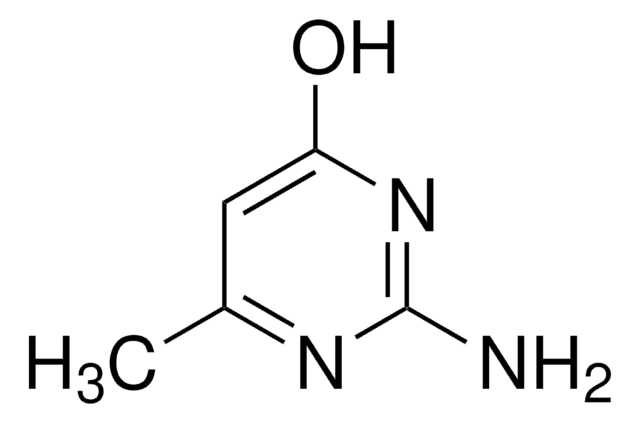
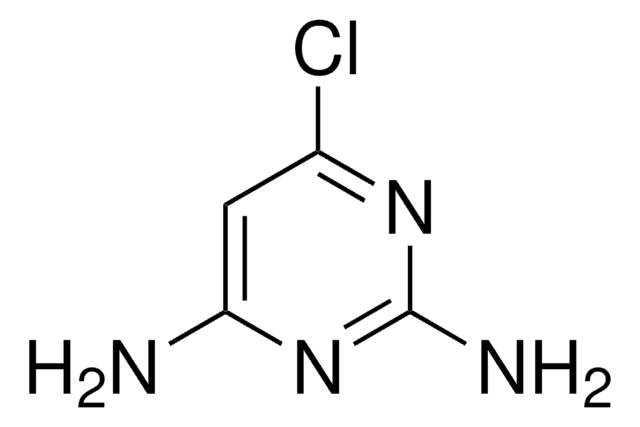
2-Amino-4-chloro-6-methylpyrimidine
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H6ClN3
Numero CAS:
Peso molecolare:
143.57
Beilstein:
114297
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
Punto di fusione
183-186 °C (lit.)
Solubilità
acetic acid: soluble 50 mg/mL, clear, colorless to faintly yellow
Gruppo funzionale
chloro
Stringa SMILE
Cc1cc(Cl)nc(N)n1
InChI
1S/C5H6ClN3/c1-3-2-4(6)9-5(7)8-3/h2H,1H3,(H2,7,8,9)
NPTGVVKPLWFPPX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2-Amino-4-chloro-6-methylpyrimidine is a nitification inhibitor.
Applicazioni
2-Amino-4-chloro-6-methylpyrimidine was used to study the influence of chlorine substitution in pyrimidine ring on proton donor ability of amino group in 2-aminopyrimidine.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Influence of chlorine-substitution in pyrimidine ring on proton donor ability in H-bond and parameters of amino group of 2-amino pyrimidine.
Borisenko VE, et al.
Vibrational Spectroscopy, 37(1), 97-109 (2005)
T Jayavarthanan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 97, 811-824 (2012-08-21)
The solid phase FTIR and FT-Raman spectra of 2-amino-4-chloro-6-methylpyrimidine (2A4Cl6MP) have been recorded in the regions 400-4000 and 50-4,000 cm(-1), respectively. The spectra have been interpreted interms of fundamentals modes, combination and overtone bands. The structure of the molecule has
J A Hutter et al.
Biochemistry, 26(7), 1969-1973 (1987-04-07)
Thiaminase I from Bacillus thiaminolyticus strain Matsukawa et Misawa is completely and irreversibly inhibited by treatment with 4-amino-6-chloro-2-methylpyrimidine. Inhibition is a time-dependent first-order process, exhibiting a half-time of 4 h at an inhibitor concentration of 5 mM. A specific active-site-directed
Effects of nitrification inhibitors on denitrification of nitrate in soil.
Bremner JM andYeomans JC.
Biology and Fertility of Soils, 2(4), 173-179 (1986)
Christer B Aakeröy et al.
Pharmaceutics, 3(3), 601-614 (2011-01-01)
In the pharmaceutical industry, co-crystals are becoming increasingly valuable as crystalline solids that can offer altered/improved physical properties of an active pharmaceutical ingredient (API) without changing its chemical identity or biological activity. In order to identify new solid forms of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.