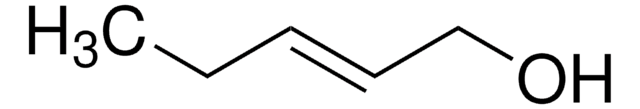
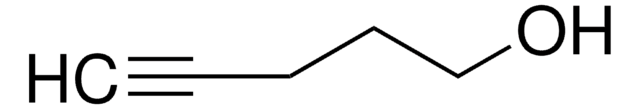
111279
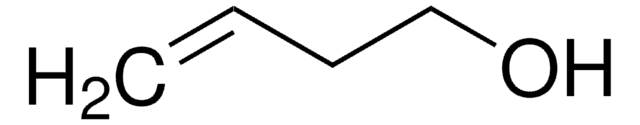
4-Penten-1-ol
99%
Sinonimo/i:
2-Allylethyl alcohol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH2=CH(CH2)3OH
Numero CAS:
Peso molecolare:
86.13
Beilstein:
1560163
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
39020310
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Indice di rifrazione
n20/D 1.429 (lit.)
P. ebollizione
134-137 °C (lit.)
Densità
0.834 g/mL at 25 °C (lit.)
Gruppo funzionale
allyl
hydroxyl
Stringa SMILE
OCCCC=C
InChI
1S/C5H10O/c1-2-3-4-5-6/h2,6H,1,3-5H2
LQAVWYMTUMSFBE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
4-penten-1-ol forms ester bond at the C terminus of the linear peptide in solution with HATU as coupling agent.
Applicazioni
4-Penten-1-ol can be used as a reactant to prepare sulfamate ester by reacting with chlorosulfonyl isocycanate (142662).The derived ester undergoes an enantioselective intramolecular azridination reaction in the presence of Cu catalyst. 4-Penten-1-ol can also be used to study the epoxidation of olefins with oxo-diperoxo tungstate(VI) complex as catalyst and bicarbonate as co-catalyst.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
118.4 °F - closed cup
Punto d’infiammabilità (°C)
48 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Enantioselective Intramolecular Copper-Catalyzed Aziridination of Sulfamates
Audrey Esteoule.et al.
Synthesis, 1251-1251 (2007)
Highly efficient epoxidation method of olefins with hydrogen peroxide as terminal oxidant, bicarbonate as a co-catalyst and oxodiperoxo molybdenum(VI) complex as catalyst.
Maiti SK, et al.
New. J. Chem., 30(3), 479-489 (2006)
Stefania Terracciano et al.
Bioorganic & medicinal chemistry, 16(13), 6580-6588 (2008-05-30)
In the recent years, we focused our attention on the cyclodepsipeptide Jaspamide 1, an interesting marine metabolite, possessing a potent inhibitory activity against breast and prostate cancer, as a consequence of its ability to disrupt actin cytoskeleton dynamics. Although its
Marina D Rvovic et al.
Journal of molecular modeling, 17(6), 1251-1257 (2010-08-17)
The mechanism of phenylselenoetherification of pent-4-en-1-ol using some bases (pyridine, triethylamine, quinoline, 2,2'-bipyridine) as catalyst was examined through studies of kinetics of the cyclization, by UV-VIS spectrophotometry. It was demonstrated that the intramolecular cyclization is facilitated in the presence of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.