100013
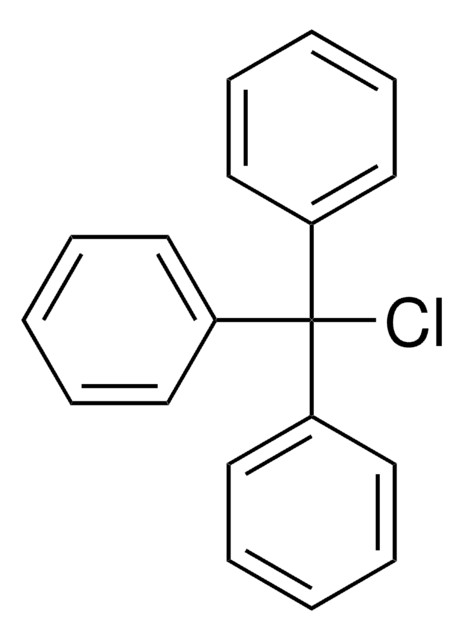
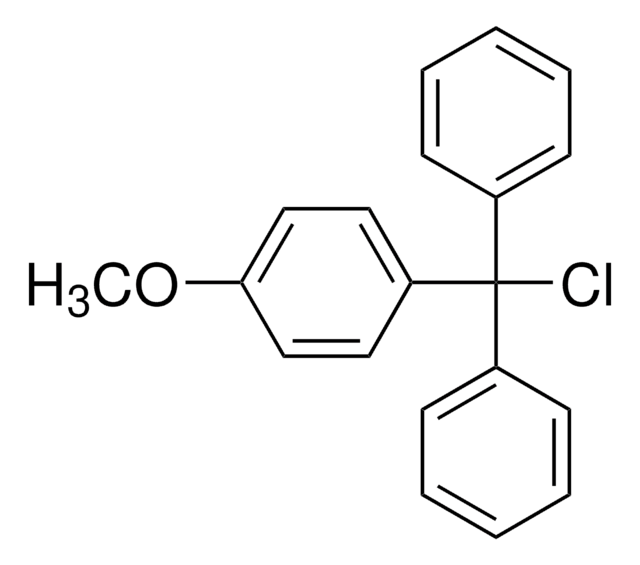
4,4′-Dimethoxytrityl chloride
95%
Sinonimo/i:
DMT-Cl
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5C(C6H4OCH3)2Cl
Numero CAS:
Peso molecolare:
338.83
Beilstein:
2471942
Numero CE:
Numero MDL:
Codice UNSPSC:
12352101
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
95%
Stato
solid
Punto di fusione
119-123 °C (lit.)
Gruppo funzionale
chloro
phenyl
Stringa SMILE
COc1ccc(cc1)C(Cl)(c2ccccc2)c3ccc(OC)cc3
InChI
1S/C21H19ClO2/c1-23-19-12-8-17(9-13-19)21(22,16-6-4-3-5-7-16)18-10-14-20(24-2)15-11-18/h3-15H,1-2H3
JBWYRBLDOOOJEU-UHFFFAOYSA-N
Descrizione generale
DMT-Cl is commonly used as a protecting group for various functional groups in organic synthesis.
Applicazioni
Hydroxyl protecting group for nucleosides and nucleotides.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Y Ueno et al.
Nucleic acids research, 21(19), 4451-4457 (1993-09-25)
The preparation of a nucleotidyl-peptide having a thymidine-5'-yl-(P-O)-serine phosphodiester bond, [H-Ala-Ser(pTpT)-Phe-OH](24) is described. After condensation between the phosphorylated peptide component and an oligonucleotide component, all protecting groups could be removed under neutral conditions without beta-elimination of the pTpT from the
Journal of the American Chemical Society, 115, 4985-4985 (1993)
T Tuschl et al.
Biochemistry, 32(43), 11658-11668 (1993-11-02)
The three guanosines of the central core of a hammerhead ribozyme were replaced by 2-aminopurine ribonucleoside, xanthosine, isoguanosine, inosine, and deoxyguanosine. These analogues were incorporated by automated solid-phase synthesis, with the exception of isoguanosine. This was introduced by ligating a
Thomas F Scott et al.
Clinical neurology and neurosurgery, 127, 86-92 (2014-12-03)
To describe a "new natural history" of multiple sclerosis (MS), characterizing three patterns of progression in Relapsing MS (RMS) patients during the "treatment era," using newly developed definitions. By utilizing our simple model we intend to predict which patients are
Yuzhe Du et al.
Molecular pharmacology, 88(2), 273-280 (2015-05-15)
Voltage-gated sodium channels are the primary target of pyrethroid insecticides. Although it is well known that specific mutations in insect sodium channels confer knockdown resistance (kdr) to pyrethroids, the atomic mechanisms of pyrethroid-sodium channel interactions are not clearly understood. Previously
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.