推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
DMSO: 20 mg/mL, clear
儲存溫度
−20°C
SMILES 字串
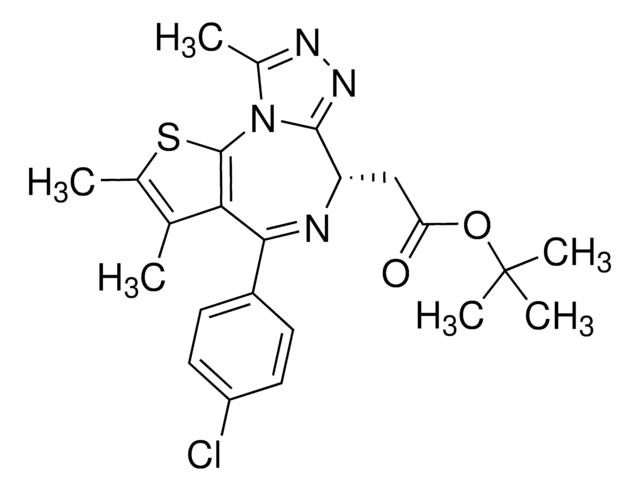
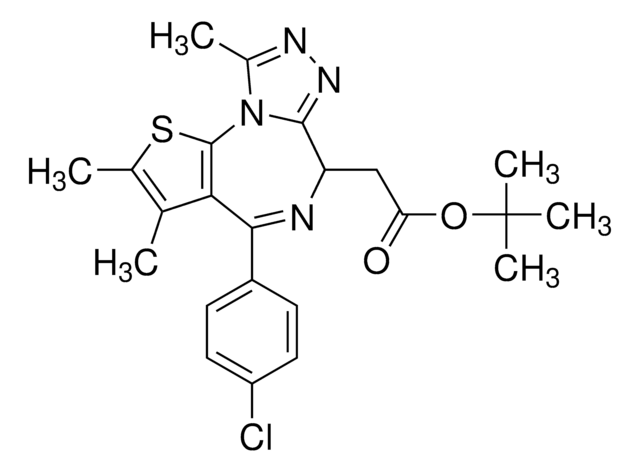
CCN1C(C2=C(SC(C(NC3CCS(CC3)(=O)=O)=N)=C2)C(C4=CC(C(F)(F)F)=CC=C4)=C1)=O
InChI
1S/C22H22F3N3O3S2/c1-2-28-12-17(13-4-3-5-14(10-13)22(23,24)25)19-16(21(28)29)11-18(32-19)20(26)27-15-6-8-33(30,31)9-7-15/h3-5,10-12,15H,2,6-9H2,1H3,(H2,26,27)
InChI 密鑰
WRUWGLUCNBMGPS-UHFFFAOYSA-N
一般說明
I-BRD9 is developed from thienopyridone scaffold. It can also be used as an identifier for BRD9 regulated gene in Kasumi-1 cells involved in oncology and immune response pathways.
生化/生理作用
I-BRD9 is a selective cellular chemical probe for bromodomain-containing protein 9 (BRD9), thought to be a member of the chromatin remodelling SWI/SNF BAF complex, which plays a fundamental role in gene expression regulation. I-BRD9 has a pIC50 value of 7.3 with greater than 700-fold selectivity over the BET family and 200-fold over the highly homologous bromodomain-containing protein 7 (BRD7) and greater than 70-fold selectivity against a panel of 34 bromodomains. For full characterization details, please visit the I-BRD9 probe summary on the Structural Genomics Consortium (SGC) website.
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
I-BRD9 is a selective cellular chemical probe for bromodomain-containing protein 9 (BRD9).
特點和優勢
I-BRD9 is an epigenetic chemical probe available through a partnership with the Structural Genomics Consortium (SGC). To learn more and view other SGC epigenetic probes, visit sigma.com/SGC.
This compound is a featured product for Gene Regulation research. Click here to discover more featured Gene Regulation products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
其他說明
I-BRD9 has been expertly reviewed and recommended by the Chemical Probes Portal. For more information, please visit the I-BRD9 probe summary on the Chemical Probes Portal website.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Natalie H Theodoulou et al.
Journal of medicinal chemistry, 59(4), 1425-1439 (2015-04-10)
Acetylation of histone lysine residues is one of the most well-studied post-translational modifications of chromatin, selectively recognized by bromodomain "reader" modules. Inhibitors of the bromodomain and extra terminal domain (BET) family of bromodomains have shown profound anticancer and anti-inflammatory properties
Structure-based design of an in vivo active selective BRD9 inhibitor.
Martin L J, et al.
Journal of Medicinal Chemistry, 59(10), 4462-4475 (2016)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持