推荐产品
化驗
≥98% (HPLC)
形狀
solid
顏色
white to off-white
溶解度
DMSO: ~12 mg/mL
H2O: insoluble
起源
GlaxoSmithKline
儲存溫度
2-8°C
SMILES 字串
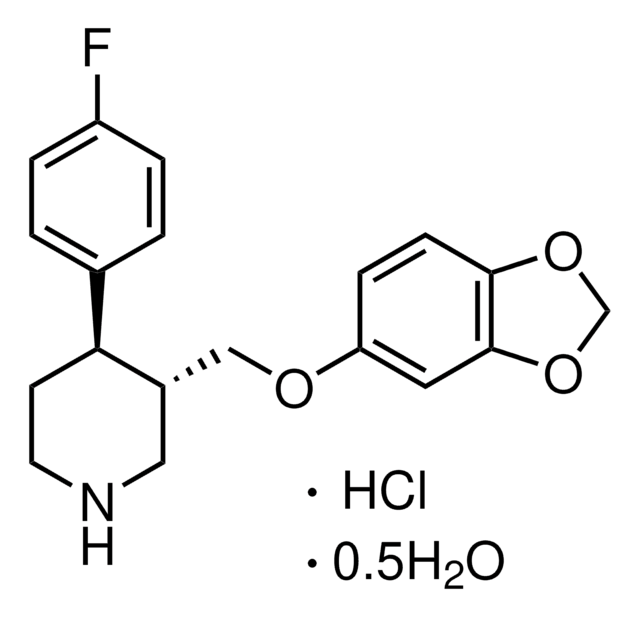
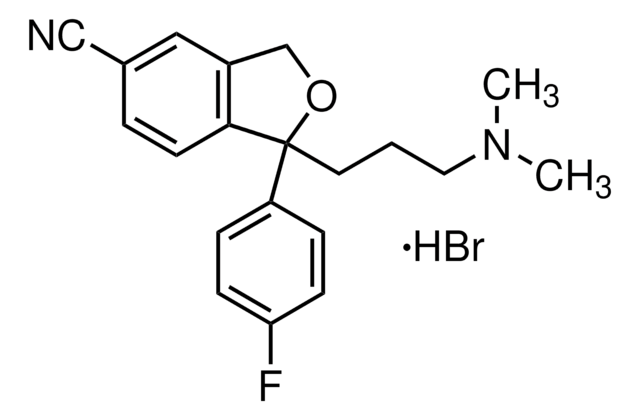
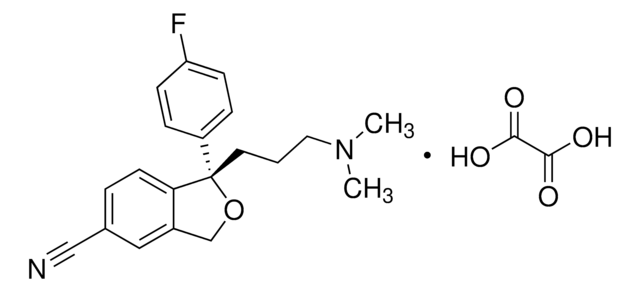
OC(=O)\C=C/C(O)=O.Fc1ccc(cc1)[C@@H]2CCNC[C@H]2COc3ccc4OCOc4c3
InChI
1S/C19H20FNO3.C4H4O4/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18;5-3(6)1-2-4(7)8/h1-6,9,14,17,21H,7-8,10-12H2;1-2H,(H,5,6)(H,7,8)/b;2-1-/t14-,17-;/m0./s1
InChI 密鑰
AEIUZSKXSWGSRU-QXGDPHCHSA-N
基因資訊
human ... SLC6A4(6532)
生化/生理作用
Paroxetine is a strong cytochrome P450 2D6 isotype (CYP2D6) inhibitor, which reduces the effectiveness of tamoxifen. This phenylpiperidine derivative inhibits clozapine metabolism. Paroxetine is used to treat social phobia, obsessive-compulsive disorder and panic disorder. It is also used to treat the premenstrual dysphoric disorder, post-traumatic stress disorder and chronic headache.
Paroxetine maleate is a selective serotonin reuptake inhibitor; antidepressant.
特點和優勢
This compound is featured on the Biogenic Amine Transporters page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by GlaxoSmithKline. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
法律資訊
Sold with the permission of GlaxoSmithKline
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Supportive Oncology E-Book (2011)
Meyler's Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions (2015)
Paroxetine: a review
Bourin M, et al.
Cns Drug Reviews, 7(1), 25-47 (2001)
Nicolas Pallet et al.
Proteomics, 13(7), 1108-1120 (2013-02-26)
The stress status of the apoptotic cell can promote phenotypic changes that have important consequences on the immunogenicity of the dying cell. Autophagy is one of the biological processes activated in response to a stressful condition. It is an important
David Germann et al.
Profiles of drug substances, excipients, and related methodology, 38, 367-406 (2013-05-15)
Paroxetine hydrochloride (3S-trans)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-piperidine hydrochloride (or (-)-(3S,4R)-(4-(p-fluorophenyl)-3-[[3,4-(methylenedioxy)-phenoxy]methyl]piperidine hydrochloride), a phenylpiperidine derivative, is a selective serotonin reuptake inhibitor. Paroxetine is indicated for the treatment of depression, generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, and social anxiety disorder. The physicochemical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门