推荐产品
生物源
synthetic (organic)
品質等級
化驗
≥99% (HPLC)
形狀
powder
溶解度
water: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
SMILES 字串
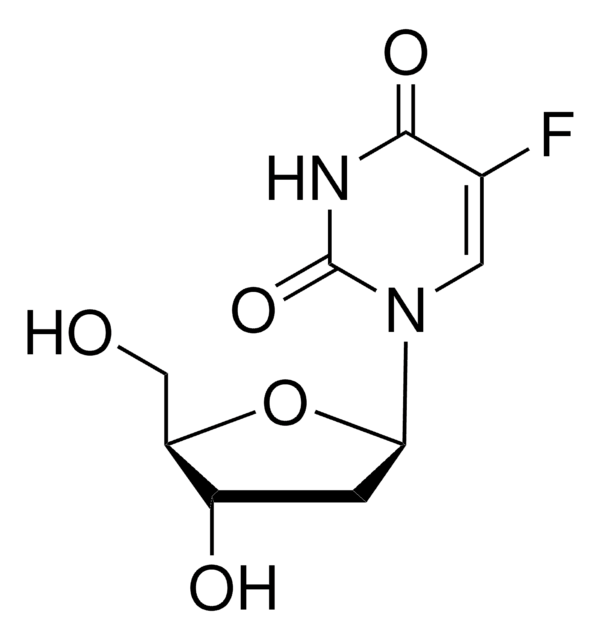
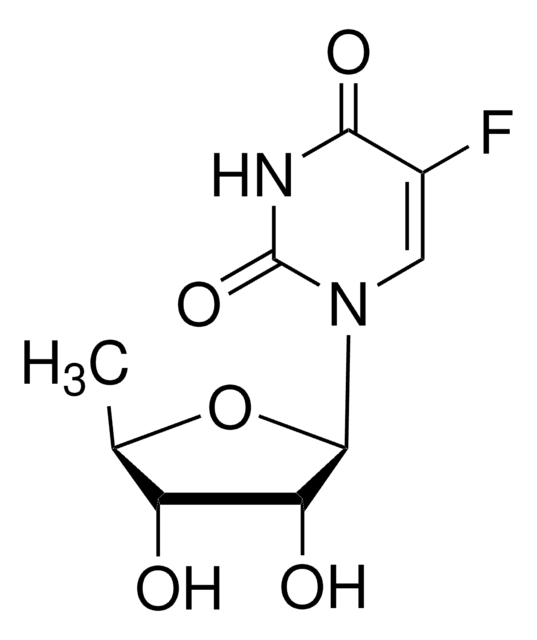
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N2C=C(F)C(=O)NC2=O
InChI
1S/C9H11FN2O6/c10-3-1-12(9(17)11-7(3)16)8-6(15)5(14)4(2-13)18-8/h1,4-6,8,13-15H,2H2,(H,11,16,17)/t4-,5-,6-,8-/m1/s1
InChI 密鑰
FHIDNBAQOFJWCA-UAKXSSHOSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
5-氟尿苷 (FUrd) 是氟嘧啶核苷类似物 和具有细胞渗透性的修饰 RNA 前体。
應用
5-氟尿苷可用于标记猪胚胎成纤维细胞 和人细胞系的活性转录位点,用于免疫细胞化学分析。 在食管鳞状细胞癌(ESCC)细胞的药物敏感性测定中,也可用于监测细胞凋亡。
生化/生理作用
5-氟尿苷(FUrd)对癌细胞有细胞毒性。Furd 通常用于氟尿嘧啶和胸腺嘧啶类似物的化学和生化比较研究。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
其他客户在看
Inhibition of RNA synthesis by 5-fluorouridine accounts for its cyto toxicity on colorectal cancer cells in vitro
Subbarayan PR, et al.
Cancer research, 65(9) (2005)
P V Sahasrabudhe et al.
Nucleic acids research, 23(19), 3916-3921 (1995-10-11)
The effects of 5-fluorouridine (FUrd) and 5-fluorodeoxyuridine (FdUrd) substitution on the stabilities of duplex RNA and DNA have been studied to determine how FUrd substitution in nucleic acids may alter the efficiency of biochemical processes that require complementary base pairing
Angelica M Bello et al.
Journal of medicinal chemistry, 52(6), 1648-1658 (2009-03-06)
A series of 6-substituted and 5-fluoro-6-substituted uridine derivatives were synthesized and evaluated for their potential as anticancer agents. The designed molecules were synthesized from either fully protected uridine or the corresponding 5-fluorouridine derivatives. The mononucleotide derivatives were used for enzyme
Edward J Miracco et al.
Journal of the American Chemical Society, 133(31), 11826-11829 (2011-07-13)
The pseudouridine synthase TruB handles 5-fluorouridine in RNA as a substrate, converting it into two isomeric hydrated products. Unexpectedly, the two products differ not in the hydrated pyrimidine ring but in the pentose ring, which is epimerized to arabinose in
Iñigo Casafont et al.
Neurotoxicity research, 17(2), 167-178 (2009-07-18)
The ubiquitin-dependent proteasome system (UPS) is the major pathway responsible for selective nuclear and cytoplasmic protein degradation. Bortezomib, a boronic acid dipeptide, is a reversible 20S proteasome inhibitor used as novel anticancer drug, particularly in the treatment of multiple myeloma
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门