推荐产品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to off-white
溶解度
DMSO: >20 mg/mL
儲存溫度
room temp
SMILES 字串
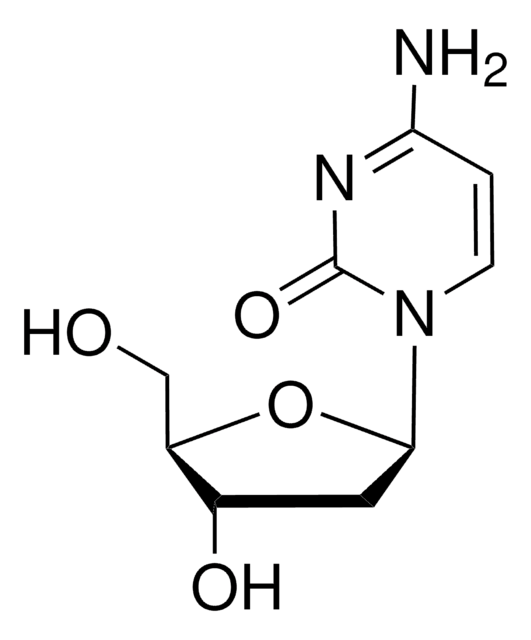
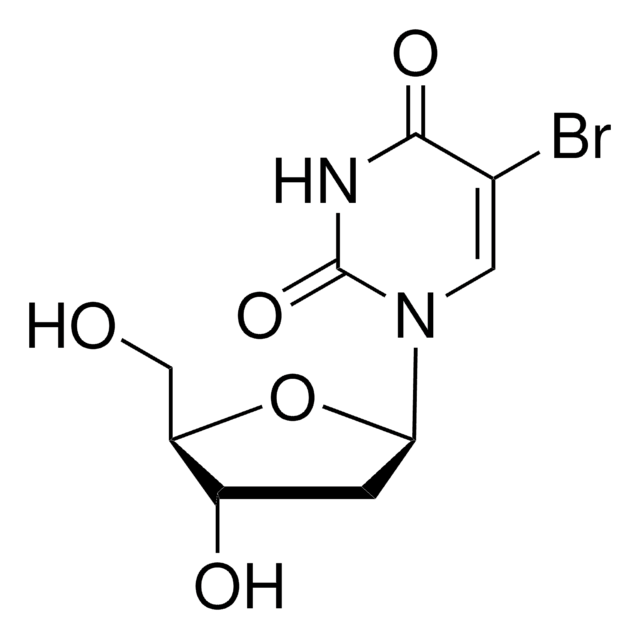
NC1=NC(=O)N(C=C1F)[C@H]2C[C@H](O)[C@@H](CO)O2
InChI
1S/C9H12FN3O4/c10-4-2-13(9(16)12-8(4)11)7-1-5(15)6(3-14)17-7/h2,5-7,14-15H,1,3H2,(H2,11,12,16)/t5-,6+,7+/m0/s1
InChI 密鑰
IDYKCXHJJGMAEV-RRKCRQDMSA-N
生化/生理作用
5-Fluoro-2′-deoxycytidine is a mechanism based DNMT (DNA cytosine-5 methyltransferase) inhibitor, that forms a covalent link with the cysteine residue in the active site of DNMT.
特點和優勢
This compound is a featured product for Gene Regulation research. Click here to discover more featured Gene Regulation products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
D A Boothman et al.
Cancer research, 47(9), 2354-2362 (1987-05-01)
The metabolic products formed and incorporated into the nucleic acids (RNA and DNA) of mice bearing Lewis lung carcinoma (LLC) following optimal doses of 5-fluorouracil (FUra), 5-fluoro-2'-deoxyuridine (FdUrd), and 5-fluoro-2'-deoxycytidine (FdCyd) coadministered with tetrahydrouridine (H4Urd), a potent inhibitor of cytidine
D J Baker et al.
Biochemical and biophysical research communications, 196(2), 864-871 (1993-10-29)
A new class of affinity labels has been developed for human DNA (cytosine-5) methyltransferases. These oligodeoxynucleotides contain 5-fluorodeoxycytidine at a mispair within the recognition motif of the human enzyme. They were not effectively recognized by bacterial methyltransferases. They can be
Avik K Ghosh et al.
Journal of the American Chemical Society, 128(13), 4172-4173 (2006-03-30)
Oxidation of a guanine nucleobase to its radical cation in DNA oligomers causes an increase in the acidity of the N1 imino proton that may lead to its spontaneous transfer to N3 of the paired cytosine. This proton transfer is
B Vandamme et al.
Human genetics, 79(4), 341-346 (1988-08-01)
The modes of action of 5-fluoro-2'-deoxyuridine (FdUrd) and 5-fluoro-2'-deoxycytidine (FdCyd) were studied in PHA-stimulated lymphocytes from normal volunteer donors and a fragile X patient. In both cell types, FdUrd and FdCyd inhibited cell proliferation at concentrations of 3 x 10(-8)
S Greer et al.
International journal of radiation oncology, biology, physics, 32(4), 1059-1069 (1995-07-15)
To extend our findings in previous radiation and biochemical studies with five rodent tumors, in which we used one and occasionally two or three irradiations. The extent of control of the EMT-6 mammary adenocarcinoma was determined using fractionated radiation (12
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门