所有图片(3)
About This Item
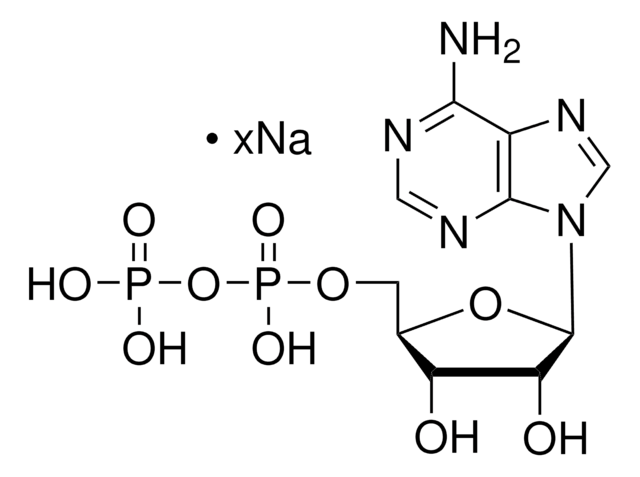
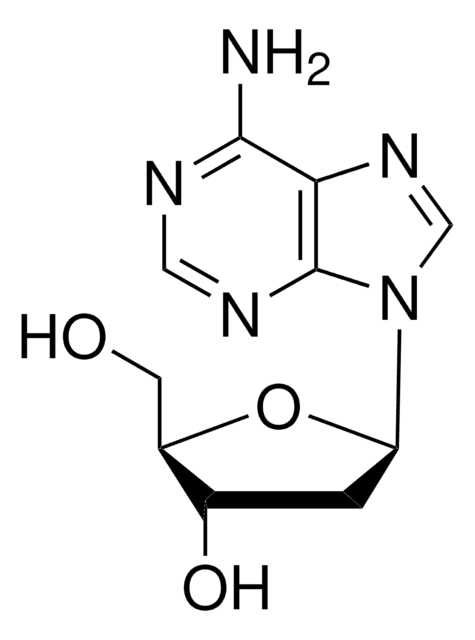
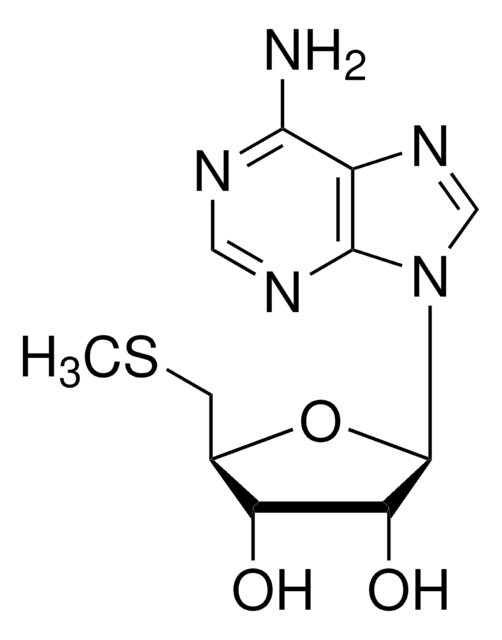
经验公式(希尔记法):
C10H15N5O9P2 · xNa+
CAS号:
分子量:
411.20 (free acid basis)
EC號碼:
MDL號碼:
分類程式碼代碼:
41106305
PubChem物質ID:
NACRES:
NA.51
推荐产品
生物源
synthetic (organic)
品質等級
化驗
≥95% (HPLC)
形狀
powder
儲存溫度
−20°C
SMILES 字串
[Na].Nc1ncnc2n(cnc12)C3CC(O)C(COP(O)(=O)OP(O)(O)=O)O3
InChI
1S/C10H15N5O9P2.Na.H/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(16)6(23-7)2-22-26(20,21)24-25(17,18)19;;/h3-7,16H,1-2H2,(H,20,21)(H2,11,12,13)(H2,17,18,19);;
InChI 密鑰
KZGAPWRJMWSNQO-UHFFFAOYSA-N
一般說明
2′-脱氧腺苷5′-二磷酸(dADP)是一种含有腺嘌呤作为核碱基的嘌呤。已知它体内嘌呤降解过程中次黄嘌呤氧化酶作用于次黄嘌呤的产物。
應用
2′-脱氧腺苷5′-二磷酸(dADP)可用于与诸如来自大肠埃希菌的多核苷酸磷酸化酶的酶合成脱氧腺苷酸寡核苷酸。dADP用作细菌聚(A)聚合酶的抑制剂。 脱氧ADP可以用作研究ATPase以及DNA和RNA聚合酶特异性的替代底物或抑制剂。
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
M P Deutscher
The Journal of biological chemistry, 253(16), 5579-5584 (1978-08-25)
Poly(A) synthesis and degradation have been examined in Escherichia coli cells made permeable to nucleotides by treatment with toluene. Although newly synthesized poly(A) is normally rapidly degraded in this system, extraction of the soluble portion of the cell effectively eliminates
Kevin N Kirouac et al.
Journal of molecular biology, 407(3), 382-390 (2011-02-08)
The ability of DNA polymerases to differentiate between ribonucleotides and deoxribonucleotides is fundamental to the accurate replication and maintenance of an organism's genome. The active sites of Y-family DNA polymerases are highly solvent accessible, yet these enzymes still maintain a
S Gillam et al.
Nucleic acids research, 2(5), 613-624 (1975-05-05)
Polynucleotide phosphorylase from Escherichia coli can be used to catalyse the addition of short tracts of deoxyadenylate residues to the 3'-termini of deoxyribooligonucleotides of the type pdAn-dN (where dN = dC, dT or dG) using dADP as donor. Similarly, the
Mahmoud Kandeel et al.
Journal of bioenergetics and biomembranes, 42(5), 361-369 (2010-08-17)
Nucleoside diphosphate kinases (NDKs) play a key role in maintaining the intracellular energy resources as well as the balance of nucleotide pools. Recently, attention has been directed to NDKs owing to its role in activating various chemotherapeutic agents. The binding
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门