推荐产品
产品名称
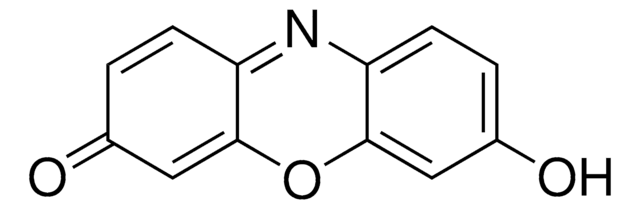
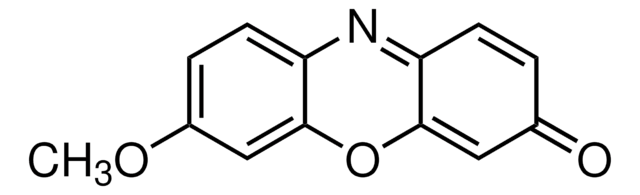
Resorufin benzyl ether, CYP450 substrate
品質等級
化驗
≥98% (TLC)
形狀
powder
溶解度
chloroform: 9.80-10.20 mg/mL, clear, orange
儲存溫度
2-8°C
SMILES 字串
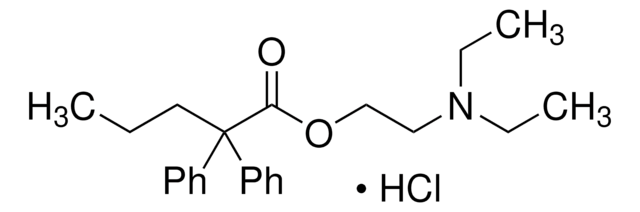
O=C1C=CC2=Nc3ccc(OCc4ccccc4)cc3OC2=C1
InChI
1S/C19H13NO3/c21-14-6-8-16-18(10-14)23-19-11-15(7-9-17(19)20-16)22-12-13-4-2-1-3-5-13/h1-11H,12H2
InChI 密鑰
XNZRYTITWLGTJS-UHFFFAOYSA-N
基底
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门