所有图片(3)
About This Item
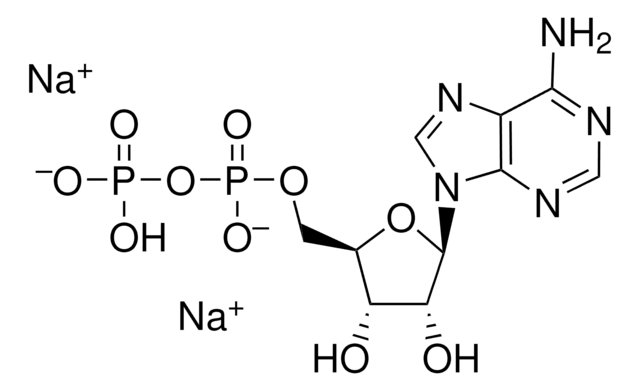
经验公式(希尔记法):
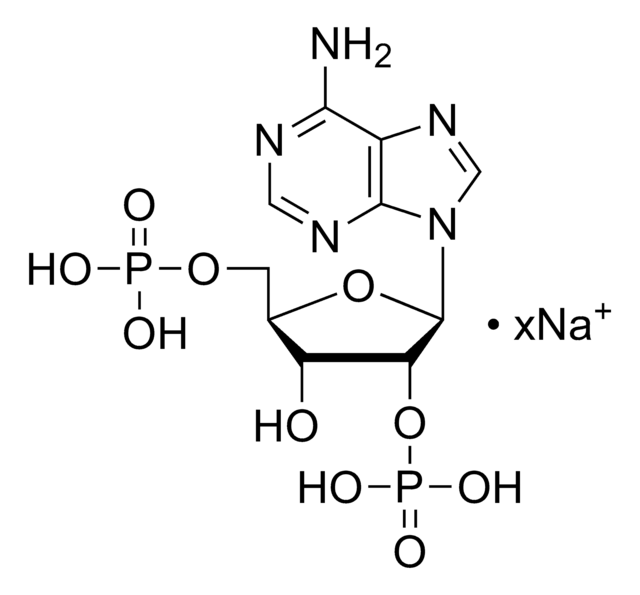
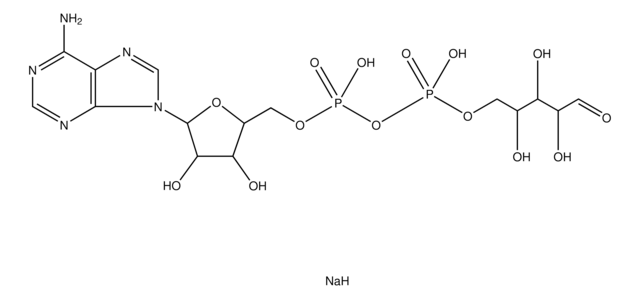
C10H13N5Na2O10P2
CAS号:
分子量:
471.16
MDL號碼:
分類程式碼代碼:
41106305
eCl@ss:
32160414
PubChem物質ID:
NACRES:
NA.51
推荐产品
生物源
synthetic (inorganic)
品質等級
化驗
≥96%
形狀
powder
溶解度
water: 25 mg/mL, clear, colorless to very faintly yellow
儲存溫度
−20°C
SMILES 字串
[Na].Nc1ncnc2n(cnc12)C3OC(COP(O)(O)=O)C(OP(O)(O)=O)C3O
InChI
1S/C10H15N5O10P2.Na.H/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(25-27(20,21)22)4(24-10)1-23-26(17,18)19;;/h2-4,6-7,10,16H,1H2,(H2,11,12,13)(H2,17,18,19)(H2,20,21,22);;
InChI 密鑰
ISROZYFZEAVMSP-UHFFFAOYSA-N
一般說明
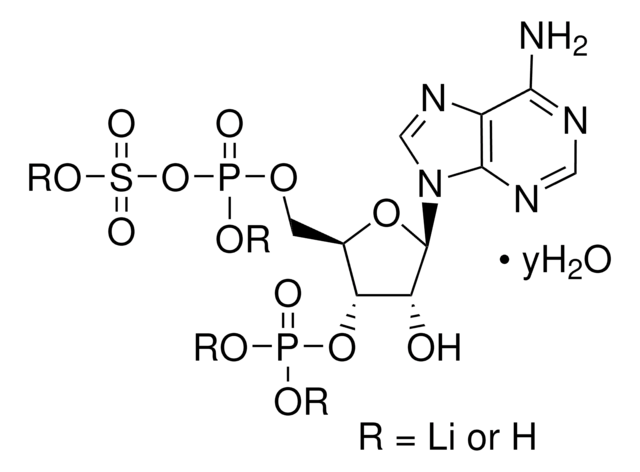
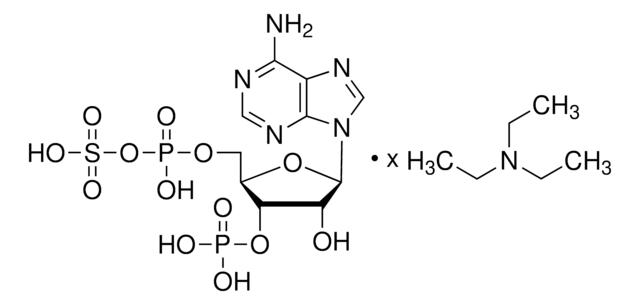
3′-磷酸腺苷5′-磷酸(PAP),为3′-磷酸化的核苷酸,几乎存在于所有生物中,是硫和脂质代谢的副产物。
應用
腺苷3′,5′-二磷酸二钠盐用于:
- 在二维薄层色谱中,在纤维素高效薄层色谱(HPTLC)板上点样
- 在酶活性试验中,研究 HOS2 / FIERY1野生型
- hos2突变体和fiery1?2突变蛋白对3′-磷酸磷腺苷5′-磷酸(PAP)的活性
- 作为标准品,定量磷酸腺苷
生化/生理作用
3′-磷酸磷腺苷5′-磷酸(PAP)能阻断细胞核和胞质溶胶中核糖核酸外切酶(XRN)的活性。 它刺激气孔关闭,在脱落酸(ABA)信号传递过程中作为第二信使。能够阻断RNA分解代谢。因此,它可以作为生理调节剂,调控聚(ADP-核糖)聚合酶1(PARP1)活性。
腺苷 3′,5′-二磷酸 (PAP)可用于研究羟类固醇磺基转移酶 (如SULT1A1、SULT2A1) 的动力学和作用机理,其是产物抑制剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Zhihao Yu et al.
Biochemistry, 47(48), 12777-12786 (2008-11-11)
Most assimilatory bacteria, fungi, and plants species reduce sulfate (in the activated form of APS or PAPS) to produce reduced sulfur. In yeast, PAPS reductase reduces PAPS to sulfite and PAP. Despite the difference in substrate specificity and catalytic cofactor
A single amino acid substitution in the Arabidopsis FIERY1/HOS2 protein confers cold signaling specificity and lithium tolerance
Xiong L, et al.
The Plant Journal, 40(4), 536-545 (2004)
Yungang Liu et al.
Chemico-biological interactions, 189(3), 153-160 (2010-12-07)
Hydroxylated metabolites of polychlorinated biphenyls (OHPCBs) interact with rat sulfotransferase 1A1 (rSULT1A1) as substrates and inhibitors. Previous studies have shown that there are complex and incompletely understood structure-activity relationships governing the interaction of rSULT1A1 with these molecules. Furthermore, modification of
Elie Toledano et al.
The Biochemical journal, 443(2), 485-490 (2012-01-14)
pAp (3'-5' phosphoadenosine phosphate) is a by-product of sulfur and lipid metabolism and has been shown to have strong inhibitory properties on RNA catabolism. In the present paper we report a new target of pAp, PARP-1 [poly(ADP-ribose) polymerase 1], a
Hayrettin Ozan Gulcan et al.
Archives of biochemistry and biophysics, 507(2), 232-240 (2010-12-29)
The cytosolic sulfotransferase hSULT2A1 is the major hydroxysteroid (alcohol) sulfotransferase in human liver, and it catalyzes the 3'-phosphoadenosine-5'-phosphosulfate (PAPS)-dependent sulfation of various endogenous hydroxysteroids as well as many xenobiotics that contain alcohol and phenol functional groups. The hSULT2A1 often displays
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门