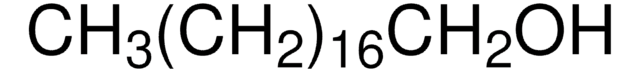推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1683606
蒸汽密度
9.7 (vs air)
蒸汽壓力
1 mmHg ( 107 °C)
API 家族
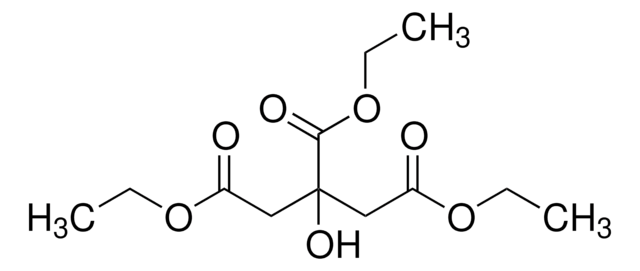
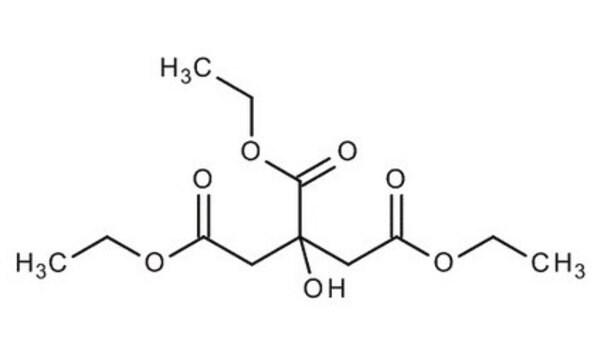
triethyl citrate
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
折射率
n20/D 1.442 (lit.)
bp
235 °C/150 mmHg (lit.)
密度
1.14 g/mL at 25 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
SMILES 字串
CCOC(=O)CC(O)(CC(=O)OCC)C(=O)OCC
InChI
1S/C12H20O7/c1-4-17-9(13)7-12(16,11(15)19-6-3)8-10(14)18-5-2/h16H,4-8H2,1-3H3
InChI 密鑰
DOOTYTYQINUNNV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Triethyl citrate is an ester of citric acid and is commonly used as a plasticizer in pharmaceutical coatings including tablets, capsules, beads and granules for masking taste. It is also used as a flavoring agent in food.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to in-house working standards.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to in-house working standards.
應用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Triethyl citrate can be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Values of analytes vary lot to lot.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA8988 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
311.0 °F - closed cup
閃點(°C)
155 °C - closed cup
其他客户在看
Oral Strip Technology: A review
Jaiswal, Hema
Indian Journal of Pharmaceutical and Biological Research, 2(2), 130-130 (2014)
Ethylcellulose--A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development
Wasilewska K and Winnicka K
Materials, 12(20), 3386-3386 (2019)
Detection and quantification of low-molecular-weight aldehydes in pharmaceutical excipients by headspace gas chromatography
Li Z, et al.
Journal of Chromatography A, 1104(1-2), 1-10 (2006)
Development and validation of a gradient HPLC method for the determination of clindamycin and related compounds in a novel tablet formulation.
Platzer DJ, White BA.
Journal of Pharmaceutical and Biomedical Analysis, 41(1), 84-88 (2006)
M R Abbaspour et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 68(3), 747-759 (2007-11-06)
One challenge in tableting of sustained-release multiparticulates is maintaining the desired drug release after compaction. The aim of this study was to design sustained-release ibuprofen tablets which upon oral ingestion rapidly disintegrate into sustained-release pellets in which the integrity of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门