推荐产品
等級
derivatization grade ((chiral))
for chiral derivatization
品質等級
化驗
≥99.0% (sum of enantiomers, TLC)
≥99.0%
形狀
powder
光學活性
[α]20/D +56±2°, c = 1% in acetone
光學純度
enantiomeric ratio: ≥99.5:0.5 (HPLC)
品質
LiChropur™
技術
HPLC: suitable
儲存溫度
2-8°C
SMILES 字串
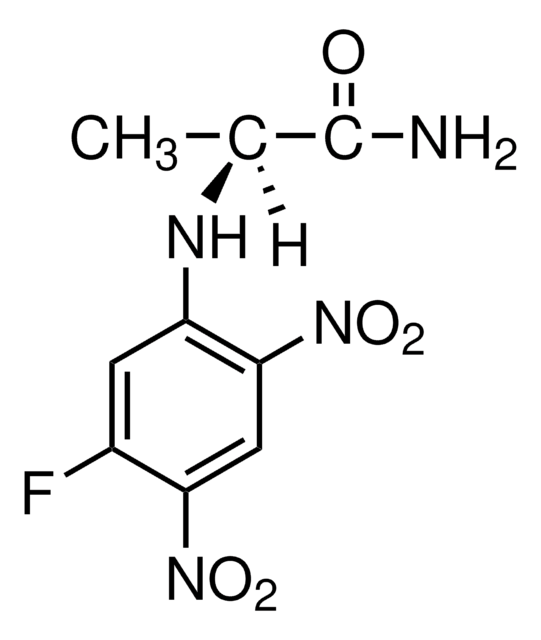
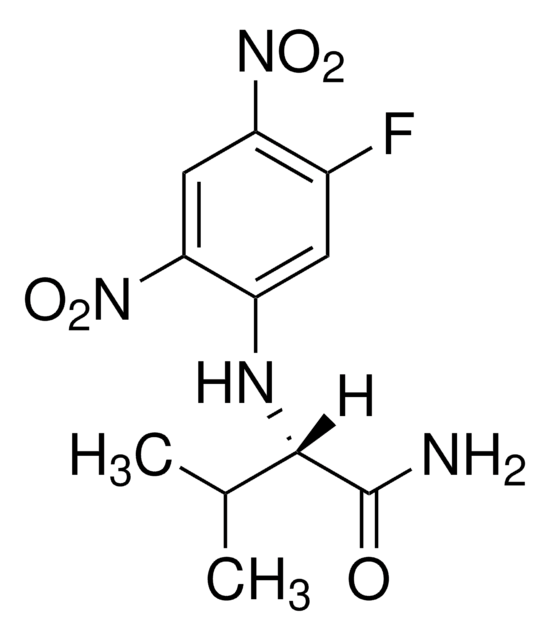
C[C@H](Nc1cc(F)c(cc1[N+]([O-])=O)[N+]([O-])=O)C(N)=O
InChI
1S/C9H9FN4O5/c1-4(9(11)15)12-6-2-5(10)7(13(16)17)3-8(6)14(18)19/h2-4,12H,1H3,(H2,11,15)/t4-/m0/s1
InChI 密鑰
NEPLBHLFDJOJGP-BYPYZUCNSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatography-electrospray ionization mass spectrometry.
Hess S
Journal of Chromatography A, 1035(2), 211-219 (2004)
R Bhushan et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 879(29), 3148-3161 (2011-07-09)
The present paper describes an updated knowledge and status on Marfey's reagent (MR), 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (FDNP-L-Ala-NH(2)). The reagent is used for pre-column derivatization of amino acids followed by HPLC separation of the diastereomers so formed. Emphasis is put on the
J G Adamson et al.
Analytical biochemistry, 202(1), 210-214 (1992-04-01)
A chromatographic assay has been developed to quantitate racemization occurring during attachment of protected amino acids to peptide synthesis resins. Acidolytic cleavage of deprotected amino acids from supports and subsequent derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (Marfey's reagent) gave diastereomers separable by
S Kochhar et al.
Analytical biochemistry, 178(1), 17-21 (1989-04-01)
Amino acids are quantitatively determined by precolumn derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide and reversed-phase high-performance liquid chromatography with photometric detection at 340 nm. Excellent chromatographic resolution of a mixture of the derivatives of 20 amino acids including proline and cystine is
A D Tran et al.
Journal of chromatography, 516(1), 241-249 (1990-09-07)
Separation of amino acid enantiomers and peptide isomers has been made possible through the use of Marfey's reagent and high-performance capillary electrophoresis (HPCE). Samples of amino acids and peptides were first derivatized with Marfey's reagent and subsequently analyzed by HPCE.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门