推荐产品
等級
purum
品質等級
化驗
≥98.0% (GC)
折射率
n20/D 1.513 (lit.)
n20/D 1.513
bp
69-71 °C/0.4 mmHg (lit.)
溶解度
chloroform: 750mg + 5 ml Chloroform mg/mL, colorless to light greenish-yellow
密度
1.337 g/mL at 20 °C
1.337 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
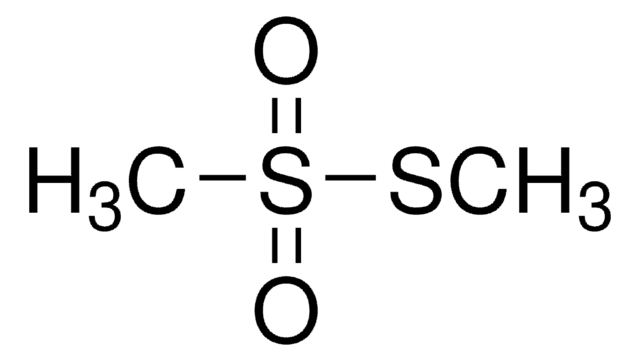
SMILES 字串
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
InChI 密鑰
XYONNSVDNIRXKZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- Modification of Thiol Enzymes: S-methyl methanethiosulfonate (MMTS) offers a unique method for the modification of thiol enzymes and redox-regulated proteins, providing potential applications in biochemical research focused on enzyme regulation and redox biology (Makarov et al., 2019).
- Sensor Development for Protease Activity: S-methyl methanethiosulfonate is used as a blocking reagent on the structural transitions of papain-like cysteine proteases, which supports its utility in sensor development, allowing for the detection and analysis of protease activity in various biological processes (Markovic et al., 2023).
- Agricultural Pathogen Control: Research evaluating S-methyl methanethiosulfonate as a late blight inhibitor highlights its potential as a broad-range toxin against plant pathogens, suggesting applications in agriculture for the management of crop diseases (Joller et al., 2020).
注意
储存时可能变为黄色
其他說明
亚甲基亚磺酰化剂有多种用途 ;对于 α-环酮的甲磺酰化 ;硫醇中的甲基二硫化物;酶的选择性、可逆性失活
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
188.6 °F - closed cup
閃點(°C)
87 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
D. Scholz
Synthesis, 944-944 (1983)
P J Britto et al.
The Biochemical journal, 389(Pt 2), 549-558 (2005-03-04)
All 20 cysteine residues are accessible to disulphide reagents in the tubulin dimer, whereas only four are accessible in taxol-stabilized microtubules. Reaction rates with disulphide reagents are a function of the reagent, are decreased by G nucleotides, and increased with
P. Laszlo et al.
The Journal of Organic Chemistry, 49, 2281-2281 (1984)
W H Briggs et al.
Journal of agricultural and food chemistry, 48(11), 5731-5735 (2000-11-23)
Thiosulfinates (TSs) have been implicated as a principle source of the antiplatelet property of raw onion and garlic juice. The in vitro responses of human platelets to dosages of four TSs were measured using whole blood aggregometry and compared by
D J Smith et al.
Biochemistry, 14(4), 766-771 (1975-02-25)
New reagents for the temporary blocking of active or accessible sulfhydryl groups of enzymes have been developed. These reagents, which are either alkyl alkanethiolsulfonates or alkoxycarbonylalkyl disulfides, rapidly and quantitatively place various RS- groups on the sulfhydryls to generate mixed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门