推荐产品
描述
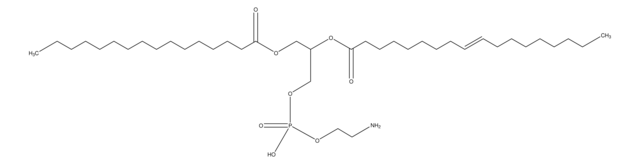
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, chloroform
化驗
>99% (TLC)
形狀
liquid
包裝
pkg of 1 × 2.5 mL (850757C-25mg)
pkg of 2 × 20 mL (850757C-1g)
pkg of 2 × 4 mL (850757C-200mg)
pkg of 5 × 4 mL (850757C-500mg)
製造商/商標名
Avanti Research™ - A Croda Brand
濃度
10 mg/mL (850757C-25mg)
25 mg/mL (850757C-1g)
25 mg/mL (850757C-200mg)
25 mg/mL (850757C-500mg)
脂質類型
cardiolipins
phospholipids
運輸包裝
dry ice
儲存溫度
−20°C
SMILES 字串
[H][C@@](COP([O-])(OCC[NH3+])=O)(OC(CCCCCCC/C=C\CCCCCCCC)=O)COC(CCCCCCCCCCCCCCC)=O
InChI
1S/C39H76NO8P/c1-3-5-7-9-11-13-15-17-18-20-22-24-26-28-30-32-39(42)48-37(36-47-49(43,44)46-34-33-40)35-45-38(41)31-29-27-25-23-21-19-16-14-12-10-8-6-4-2/h17-18,37H,3-16,19-36,40H2,1-2H3,(H,43,44)/b18-17-
InChI 密鑰
FHQVHHIBKUMWTI-ZCXUNETKSA-N
應用
包裝
法律資訊
也與該產品經常一起購買
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
標靶器官
Central nervous system, Liver,Kidney
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 850757C-200MG | 4061837792748 |
| 850757C-1G | 4061837792731 |
| 850757C-25MG | 4061837792755 |
| 850757C-500MG | 4061837792762 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持